Reprinted with permission from University of Toronto Press (https://utpjournals.press).
Shazia Damji PharmD, ACPR1, Jerrold Perrott BSc Pharm, ACPR, PharmD1, Salomeh Shajari BSc1, Jennifer Grant MD, FRCPC1, Titus Wong MD, MHSc, FRCPC1, Megan Harbin BSc Pharm, ACPR, PharmD2
1Department of Pharmacy, Vancouver General Hospital, Vancouver, British Columbia, Canada
2Department of Pharmacy, Royal Inland Hospital, Kamloops, British Columbia, Canada
Corresponding author:
Shazia Damji
Department of Pharmacy
Vancouver General Hospital
899 W 12th Avenue
Vancouver, British Columbia
V5Z 1M9 Canada
Tel: 604-875-4111 | Email: shazia.damji@vch.ca
Contributors:
Conceptualization, S Damji, J Perrott, J Grant, M Harbin; Methodology, S Damji, J Perrott, T Wong, M Harbin; Validation, S Shajari, T Wong, M Harbin; Formal Analysis, S Damji, S Shajari, M Harbin; Investigation, S Damji, J Perrott, M Harbin; Data Curation, S Damji, S Shajari, J Grant; Writing – Original Draft, S Damji, J Grant; Writing – Review & Editing, S Damji, J Perrott, J Grant, S Shajari, T Wong, M Harbin; Visualization, S Damji, S Shajari; Supervision, J Perrott, M Harbin; Project Administration, J Perrott, J Grant, M Harbin.
ABSTRACT
Background: Among hospitalized patients, a 48-hour window from time of hospitalization defines nosocomial infections and guides empiric antibiotic selection. This time frame may lead to overuse of broad-spectrum antibiotics. Our primary objective was to determine the earliest and median time since hospital admission to acquire antibiotic-resistant pathogens among patients admitted to the intensive care unit (ICU) of an academic, tertiary care hospital.
Methods: Retrospective chart review was conducted for adult patients admitted to the ICU from home or another hospital within the same health authority in 2018, to identify the time to acquisition of hospital-associated pathogens: methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, extended-spectrum beta-lactamase (ESBL)–producing Enterobacterales, non-ESBL ceftriaxone-resistant Enterobacterales, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. Patients transferred from hospitals outside the health authority, admitted to ICU after 14 days of hospitalization, who were solid organ or bone marrow transplant recipients, or who were otherwise immunocompromised were excluded.
Results: In 2018, 1,343 patients were admitted to this ICU; 820 met the inclusion criteria. Of these, 121 (14.76%) acquired a hospital-associated pathogen in the ICU. The probability of isolating a hospital-associated pathogen by 48 hours of hospital admission was 3%. The earliest time to isolate any of these pathogens was 29 hours, and the median was nine days (interquartile range [IQR] 3.8-15.6 days).
Conclusions: Most patients (85.3%) in this ICU never acquired a hospital-associated pathogen. The median time to acquire a hospital-associated pathogen among the remaining patients suggests that initiating empiric broad-spectrum antibiotics on the basis of a 48-hour threshold may be premature.
KEYWORDS
antibiotics, antimicrobial stewardship, critical care, healthcare-associated infections, nosocomial infections
ABSTRAT
Historique : Chez les patients hospitalisés, une fenêtre de 48 heures après le moment de l’hospitalisation définit les infections nosocomiales et oriente la sélection d’antibiotiques empiriques. Cette période peut favoriser la surutilisation d’antibiotiques à large spectre. L’objectif primaire de l’étude visait à déterminer la période la plus courte et la période médiane à compter de l’admission pour que les patients admis en soins intensifs à partir d’un hôpital universitaire de soins tertiaires contractent des agents pathogènes antibiorésistants.
Méthodologie : Les chercheurs ont procédé à un examen rétrospectif des dossiers des patients adultes admis en soins intensifs à partir de la maison ou d’un autre hôpital de la même autorité sanitaire en 2018, afin de déterminer la période avant de contracter des agents pathogènes associés au milieu hospitalier : Staphylococcus aureus résistant à la méthicilline, entérocoque résistant à la vancomycine, Enterobacterales producteurs de bêta-lactamases à spectre élargi (BLSE), Enterobacterales résistant à la ceftriaxine non producteurs de BLSE, Pseudomonas aeruginosa et Stenotrophomonas maltophilia. Ont été exclus les patients transférés d’un hôpital hors de l’autorité sanitaire, admis en soins intensifs plus de 14 jours après l’hospitalisation, receveurs d’un organe plein ou de moelle osseuse ou autrement immunodéprimés.
Résultats : En 2018, 1 343 patients ont été admis en soins intensifs, dont 820 respectaient les critères d’inclusion. De ce nombre, 121 (14,67 %) ont contracté un agent pathogène en soins intensifs. La probabilité d’isoler un tel agent dans les 48 heures suivant l’admission en milieu hospitalier s’élevait à 3 %. Ces agents pathogènes ont été isolés au plus tôt 29 heures après l’hospitalisation, et au bout d’une période médiane de neuf jours (plage interquartile [PIQ] 3,8 à 15,6 jours).
Conclusions : La plupart des patients (85,3%) de cette unité de soins intensifs n’ont jamais contracté d’agent pathogène associé au milieu hospitalier. Selon la période médiane avant d’acquérir un tel agent pathogène chez les autres patients, il serait prématuré d’entreprendre une antibiothérapie à large spectre au seuil de 48 heures.
MOTS-CLÉS
antibiotiques, gestion des antimicrobiens, infections associées aux soins de santé, infections nosocomiales, soins intensifs
INTRODUCTION
Compared with community-acquired infections, hospital-associated infections show greater antimicrobial resistance, with trends toward greater prevalence of gram-negative bacteria, methicillin resistance, and extended-spectrum beta-lactamase (ESBL) production, and they may be associated with higher morbidity, mortality, and healthcare costs [1,2]. Hospital-associated pathogens are assumed to include multi-drug-resistant gram-negative bacteria, including Enterobacterales, Acinetobacter, and Pseudomonas spp, as well as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) [2]. As a result, broad-spectrum empiric antimicrobial regimens are recommended for infections that are thought to be hospital associated until susceptibilities are available.
In practice, many institutions use the definition of nosocomial acquisition as occurring between 48 and 96 hours after hospital admission [3–6]. Despite the fact that this is a surveillance definition, many clinicians change prescribing patterns assuming that patients are likely to be colonized with a multi-drug-resistant organism after that time frame. However, consistent evidence is lacking to support the idea that broader empiric antibiotic therapy is necessary among hospitalized patients after this short time frame. As such, patients are often empirically prescribed broad-spectrum antibiotics (e.g., beta-lactam or beta-lactamase inhibitors and carbapenems) that may not be necessary for all patients.
Overuse of broad-spectrum antibiotics can contribute to development of antimicrobial resistance and Clostridioides difficile infections [7]. The development of antimicrobial-resistant organisms is a major public health concern; hence, the administration of appropriate empiric antibiotic agents is critical [8]. Patients admitted to the intensive care unit (ICU) have several comorbidities and severe acute pathologies that put them at higher risk for infection and mortality. The majority of ICU patients have invasive devices in place, such as mechanical ventilators, central venous catheters, and urinary catheters, that may increase their risk for acquiring infection [4]. Given these risk factors, ICU patients are often prescribed broad-spectrum empiric antimicrobials early on during their hospitalization. Identifying the timing and pattern of acquisition of hospital-associated pathogens among these high-risk patients will inform future practice patterns for empiric antibiotic use in the ICU.
This study was designed to delineate at what point changing empiric therapy from narrower to broader spectrum antibiotics would be reasonable in a low-resistance environment. Our aims were to determine the rates of isolation of multi-drug-resistant hospital-associated pathogens at the time of ICU admission, the time since hospitalization to acquisition of multi-drug-resistant pathogens, and the most common sites of isolation of hospital-associated pathogens. Our study was conducted in an academic tertiary care hospital with a 34-bed ICU. We hypothesized that the time frame to colonization or infection with a multi-drug-resistant hospital-associated pathogen in the ICU would be longer than 72 hours.
METHODS
Definitions
For the purpose of this study, we use the following definitions: Acquisition is defined as the identification of a multi-drug-resistant organism in a patient not previously known to have been colonized or infected. Colonization is defined as the identification of a multi-drug-resistant organism not thought to be causing clinical disease or not requiring therapy. Infection is defined as the identification of a multi-drug-resistant organism thought to be causing disease or that was targeted with antimicrobial therapy.
Study population
We performed a retrospective chart review of patients admitted to the ICU of an academic tertiary care hospital between January 1 and December 31, 2018. Data were collected from the hospital’s electronic medical record and medical microbiology database. We collected demographic data, date and time of hospital admission and discharge, and date and time of ICU admission and discharge, along with microbiological data, including sources of positive microbiological samples, the microorganism isolated and its sensitivities, and the date and time of sample collection. Data collection was conducted by one member of the study team, and 10% of records were double checked by a supervising member of the study team to ensure consistency.
The study inclusion criteria were as follows: adult patients (aged 18 years or older) admitted to the ICU from the emergency department, transferred from another ward, or transferred from another hospital using the same electronic medical record system. If a patient had been admitted to the ICU more than once during the study period, we included the first ICU stay during the 2018 calendar year only.
The study exclusion criteria were as follows: patients admitted to the ICU after at least 14 days of hospital admission, solid organ transplant recipients, bone marrow transplant recipients, and otherwise immunocompromised patients. For the purpose of this study, we defined immunocompromised patients as those who met any of the following criteria: [1] taking any of the following medications: corticosteroids equivalent to prednisone 20 mg by mouth daily for three or more weeks, any monoclonal antibody, any biologic, tacrolimus, cyclosporine, sirolimus, azathioprine, or mycophenolate mofetil [9]; [2] actively undergoing chemotherapy; [3] diagnosed with HIV with a CD4 count of less than 200 cells/mm3; or [4] neutropenic, defined as having an absolute neutrophil count of less than 1,500 cells/µL [10].
Using microbiology reports from the past 1 year in our electronic medical record system, patients were assessed for the presence of MRSA, VRE, ESBL-producing Enterobacterales, non-ESBL ceftriaxone-resistant Enterobacterales, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. P. aeruginosa and S. maltophilia isolates were included as hospital-associated pathogens regardless of susceptibility and antibiotic resistance. Pathogens were recorded as ESBL-producing or non-ESBL-producing ceftriaxone-resistant Enterobacterales as reported in the culture and sensitivity report. Time of first collection of these hospital-associated pathogens was considered the time of acquisition.
If a patient acquired more than one of the listed hospital-associated pathogens during their ICU admission, each isolated pathogen was recorded and analyzed independently. Samples of interest included blood, respiratory, urine, and deep wound; any additional samples such as peritoneal fluid or cerebrospinal fluid were categorized as ‘other.’ Time to acquisition was calculated as the difference between the time of hospital admission and the time of sample acquisition [11].
Each value was rounded to the nearest 15 minutes. Time since hospital admission was used, rather than time since ICU admission, because this is the time frame usually considered when selecting empiric antibiotics.
Patients who were colonized or infected with a hospital-associated pathogen before ICU admission were identified by screening microbiological results from 1 year before hospital admission until 24 hours after ICU admission. Any hospital-associated pathogen identified during this time frame was treated as prior colonization and not as an ICU-acquired pathogen.
Study end points
The primary outcome was to determine the earliest and median time since hospital admission to acquisition of a hospital-associated pathogen among ICU patients.
The secondary outcomes were as follows: (1) to describe the sites from which these microbiological samples were isolated, (2) to determine how many patients were known to have previously isolated a hospital-associated pathogen upon ICU admission, and (3) to determine the median time to acquisition of each specific hospital-associated pathogen.
Statistical analysis
The patterns of acquisition of hospital-associated pathogens were analyzed using descriptive statistics and Kaplan-Meier analysis. Demographic data are described using means and standard deviations. Outcome data such as times to acquisition of hospital-associated pathogens are presented as medians with interquartile ranges to reduce the chance of our outcomes being swayed by outliers. A Kaplan-Meier analysis was used to analyze the probability of acquiring each hospital-associated pathogen among ICU patients.
Study oversight and ethics approval
The protocol was approved by the institutional review boards of the affiliated hospital and health authority review boards.
Consent
This was a retrospective chart review and did not involve any patient interaction; it was judged minimal risk by our institution’s ethics review board.
RESULTS
Study population
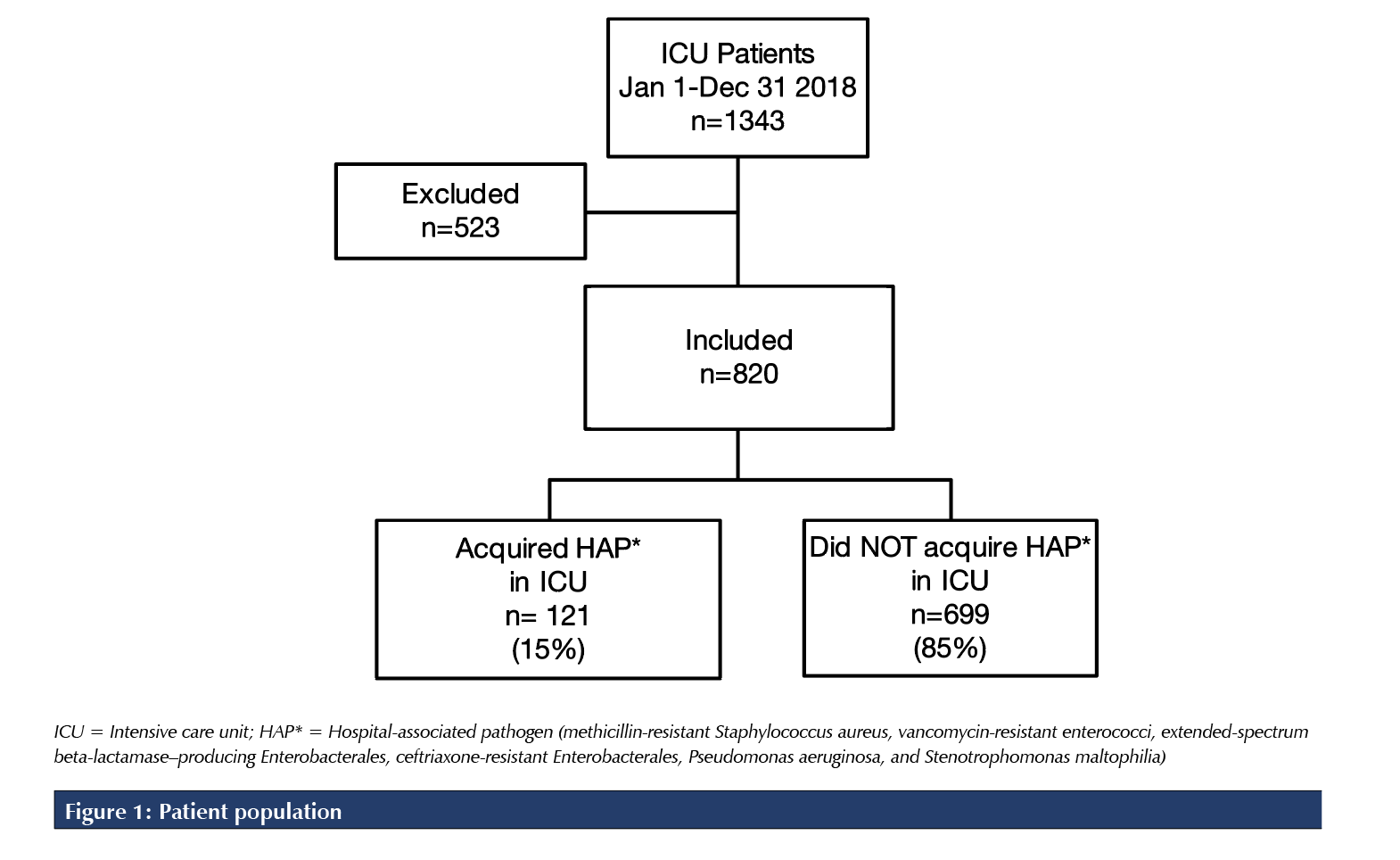
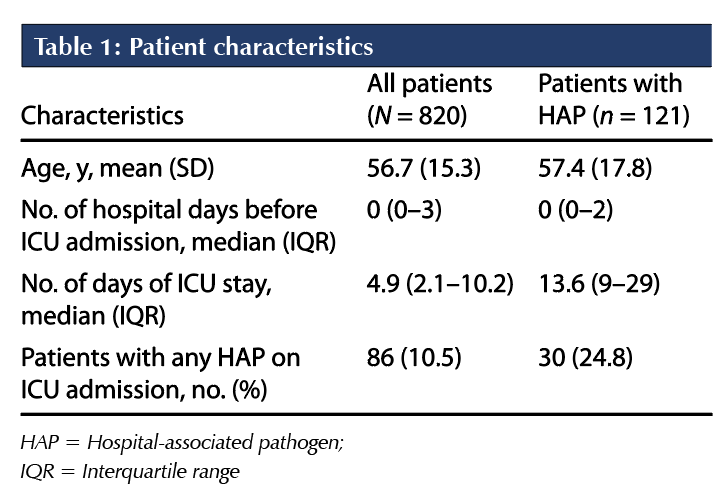
A total of 1,343 patients were admitted to the ICU between January 1 and December 31, 2018. Of these, 820 patients met the study inclusion criteria. Of the 523 patients excluded from this study, 280 were transferred from another hospital, 140 were solid organ or bone marrow transplant recipients, 58 were hospitalized 14 days or more before ICU admission, and 45 were immunocompromised (Figure 1). The mean age was 56.7 years, and the median duration of hospitalization before ICU admission was zero days (interquartile range 0-3 days). The median ICU length of stay was 4.9 days for all included patients and 13.6 days for the subset of patients who acquired a hospital-associated pathogen (Table 1).
Primary outcome
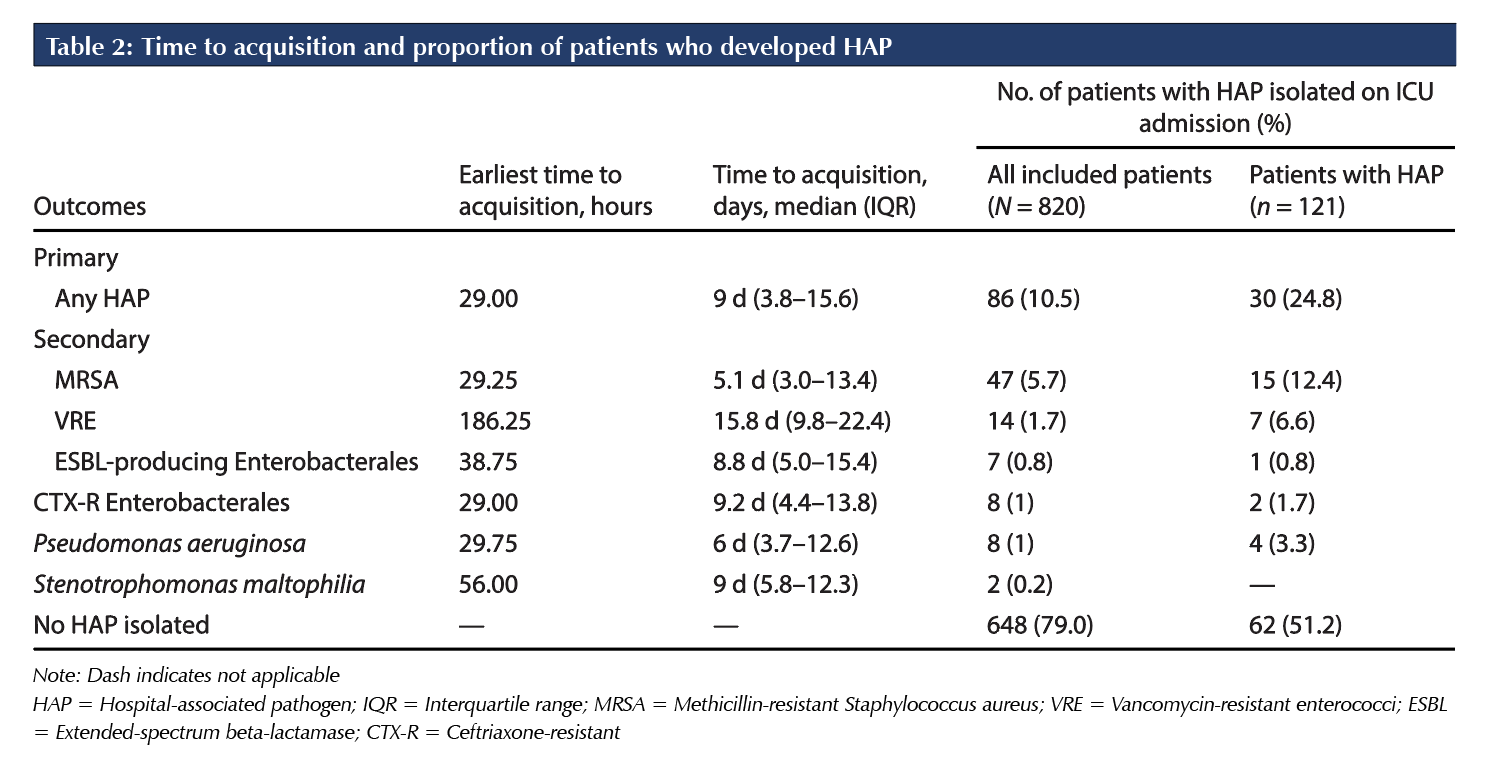
Of the 820 patients included in the study, 121 (14.8%) acquired at least one hospital-associated pathogen while admitted to the ICU. A total of 174 positive isolates were collected: 42 MRSA, 36 ESBL-producing Enterobacterales,
35 P. aeruginosa, 28 non-ESBL ceftriaxone-resistant Enterobacterales, 18 VRE, and 15 S. maltophilia.
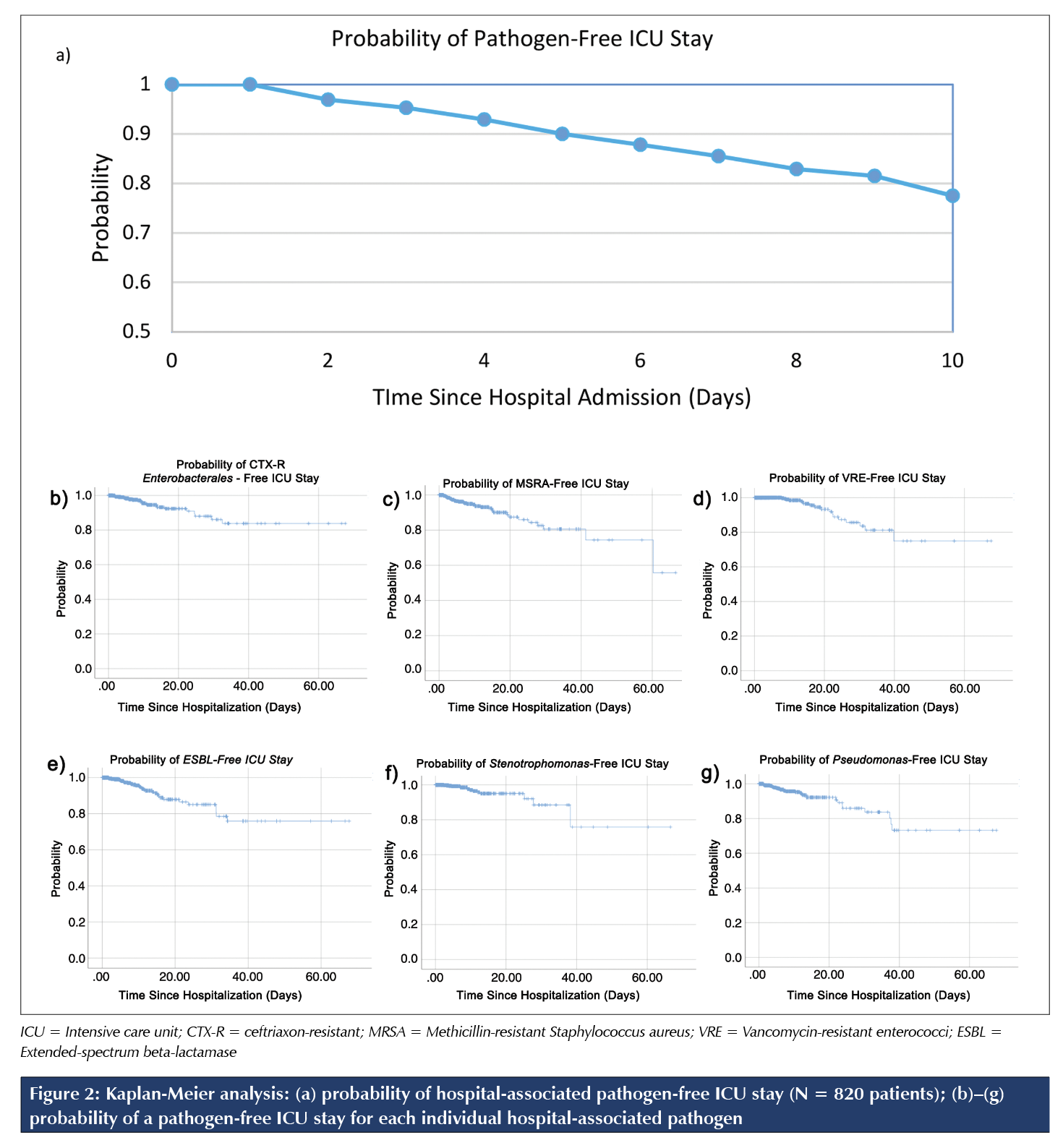
The earliest time to acquire any hospital-associated pathogen was 29 hours after hospital admission. The median time to acquire any hospital-associated pathogen was nine days (IQR 3.6–15.8 d). A Kaplan-Meier survival analysis (Figure 2) was conducted to illustrate the probability of acquiring a hospital-associated pathogen in the ICU over time. The analysis shows that the probability of acquiring a hospital-associated pathogen in the ICU within 48 hours of hospital admission is approximately 3%. Kaplan-Meier analyses for each individual hospital-associated pathogen are displayed in Figures 2b–2g.
Secondary outcomes
The most common site of isolation of hospital-associated pathogens among these ICU patients was respiratory, followed by both blood and urine and, last, deep wound. Other sources of isolation include pleural fluid, superficial wound, and tissue or bone.
Among the 820 patients admitted to the ICU, 46 were identified as having a hospital-associated pathogen within 24 hours of ICU admission; these were likely acquired before ICU admission. Eighteen patients isolated cultures of MRSA, 8 isolated non-ESBL ceftriaxone-resistant Enterobacterales, eight isolated P. aeruginosa, seven isolated ESBL-producing Enterobacterales, three isolated VRE, and two isolated S. maltophilia. Forty patients tested positive for a hospital-associated pathogen on nasal swab, perirectal swab, or both; 29 patients were positive with MRSA; and 11 patients were positive with VRE.
In the subset of 121 patients who acquired a hospital-associated pathogen in the ICU, 12 had isolated a different hospital-associated pathogen on ICU admission. Four had previously acquired MRSA; 4, P. aeruginosa; 2, non-ESBL ceftriaxone-resistant Enterobacterales; 1, ESBL-producing Enterobacterales; and 1, VRE; none acquired S. maltophilia before ICU admission (Table 4). Eighteen of these patients tested positive for a hospital-associated pathogen on nasal or perirectal swab on ICU admission; 11 were positive with MRSA and seven were positive with VRE.
Among patients who acquired a hospital-associated pathogen, the median time since hospital admission to acquire each of the hospital-associated pathogen was as follows: 5.1 days for MRSA, 6.0 days for Pseudomonas aeruginosa, 8.8 days for ESBL-producing Enterobacterales, 9.0 days for Stenotrophomonas maltophilia, 9.2 days for non-ESBL ceftriaxone-resistant Enterobacterales, and 15.8 days for VRE (Table 2). However, the majority (85.2%) of patients in the ICU did not acquire any hospital-associated pathogens.
DISCUSSION
Broad-spectrum antibiotics are initiated empirically with patients who were admitted to the hospital at least 48 hours before the onset of signs or symptoms or if there has been previous isolation of a multi-drug-resistant organism or recent antibiotic use [12-14]. Use of broader-spectrum antibiotics is correlated with the emergence of antimicrobial resistance [8, 15-17] as well as increased risk of mortality [18]. Identifying the pattern and timing of acquisition of hospital-associated pathogens will help inform future antibiotic prescribing practices to reduce unnecessary spectrum when possible.
In our study population of 820 patients, 14.8% acquired a hospital-associated pathogen in the ICU, with a median time to acquisition of 9 days. This finding contrasts the current practice of implementing broad-spectrum antibiotics empirically after 48 hours of hospitalization. Through Kaplan-Meier analysis, we determined that the probability of isolating a new hospital-associated pathogen by 48 hours of hospitalization is 3%. Our findings may refine the time frame in which broader spectrum antibiotics should be considered for immunocompetent patients admitted to the ICU.
Our data show that MRSA and P. aeruginosa were more likely to be isolated earlier (5 and 6 d, respectively) than non-ESBL ceftriaxone-resistant Enterobacterales or VRE (9 and 16 d, respectively). Similar to the findings of other studies [8, 19], MRSA was identified as the most commonly acquired hospital-associated pathogen and S. maltophilia was identified as the least common. The respiratory tract was the most common site of isolation, with bacteremias and urinary cultures the next most common isolation source and deep wound cultures the least common. These results parallel those of a study conducted in 1,200 ICUs globally in 2017 that concluded that the lungs were the most common site of infection, followed by the abdomen, bloodstream, and urinary tract [4]. Therefore, our results can be most easily applied to hospital-acquired and ventilator-associated pneumonias and less so to wound infections.
The overall median length of ICU stay was 4.9 days, whereas the median length of ICU stay among patients who acquired a hospital-associated pathogen was 13.6 days. This is consistent with previous work suggesting that ICU length of stay is a risk factor for acquiring more resistant pathogens. However, it is equally plausible that acquisition of a hospital-associated pathogen may lead to an increased length of ICU stay.
We excluded patients admitted to the hospital at least 14 days before ICU admission because our goal was to evaluate the minimum and median time to acquire hospital-associated pathogens in the ICU, and we were concerned that patients with long prior admissions would be more likely to have acquired the pathogen on the ward. We also assumed that patients admitted for more than 14 days would be likely to have microbiology on record to help guide empiric therapy in the case of deterioration. We also excluded hospital-associated pathogens that were present before ICU admission or that were isolated within 24 hours of ICU admission because these pathogens would be considered to have been acquired before ICU admission and would not have been representative of newly acquired pathogens during this present ICU stay. However, it is still possible that community-acquired pathogens may have been captured in this study; for example, if a patient did not have any prior microbiological data collected, it would not have been possible to distinguish between a community- or a hospital-acquired pathogen. This risk in particular applies to MRSA, which has been increasingly prevalent in the community; approximately 25% of MRSA infections in British Columbia are community acquired [20].
We consciously excluded immunocompromised patients from this study; therefore, our results and timelines cannot be applied to this population, for whom broad-spectrum antibiotics are used routinely for reasons other than hospital-associated pathogens. This may increase the external validity of our results to other ICUs that may not serve a high proportion of immunocompromised and post-transplant patients.
Given that clinicians refer to the time since hospital admission rather than time since ICU admission when making these decisions, we did not assess time since ICU admission. We assessed the most commonly isolated hospital-associated pathogens and may not have captured all multi-drug-resistant organisms. However, in our context, other organisms are rarely identified and are therefore of minor interest. By using microbiological data, we assumed that the time of collection of the microbiological cultures aligned with the time the pathogen was acquired. This may overestimate the time to acquisition by missing the actual emergence of the pathogen, but it reflects real-world practice. Moreover, focusing on microbiological data may have led us to include clinically irrelevant isolates, such as asymptomatic bacteriuria. We were unable to include microbiological results collected in other healthcare facilities, recent antibiotics, and recent hospitalizations, so we may have overcounted previously known pathogens.
Because of the scope of this study, we did not characterize the difference in time frame and prevalence of multi-drug-resistant hospital-associated pathogens acquired in the community, in the non-ICU ward of the hospital, and in the ICU of this hospital. This comparison can be explored in a subsequent study.
CONCLUSIONS
In our study at an academic tertiary care ICU, the probability of acquiring a hospital-associated pathogen by 48 hours of hospitalization was 3%. The earliest time to acquire a hospital-associated pathogen was 29 hours, and the median time was nine days. Therefore, use of empiric broad-spectrum antibiotics as opposed to narrower-spectrum antibiotics to treat a new infection acquired in the ICU at a threshold of 48 hours of hospitalization is likely unnecessary for many ICU patients. Clinical presentation, past microbiological cultures, and previous antibiotic use should be considered in addition to time since hospitalization when recommending empiric antibiotics.
These data are representative of our experience in a relatively low-resistance environment. Further research is needed to assess the generalizability of our findings, as well as whether specific risk factors can identify those patients more at risk for acquiring hospital-associated pathogens. Future studies could also assess the pattern of acquisition of hospital-associated pathogens among immunocompromised critically ill patients and patients admitted to a general inpatient ward.
REFERENCES
1. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and healthcare costs. Clinical Infectious Diseases. 2006;42(Supplement 2):S82–9. https://doi. org/10.1086/499406. Medline:16355321
2. Mammina C, Geraci DM, Saporito L et al. Healthcare associated pathogens in a changing world. Ital J Pediatr 2014;40(Supplement 1):A5. https://doi. org/10.1186/1824-7288-40-S1-A5
3. Schwab F, Geffers C, Behnke M, Gastmeier P. ICU mortality following ICU-acquired primary bloodstream infections according to the type of pathogen: a prospective cohort study in 937 Germany ICUs (2006-2015). PloS One. 2018;13(3):e0194210. https://doi.org/10.1371/journal.pone.0194210. Medline:29518133
4. Adrie C, Garrouste-Orgeas M, Essaied WI, et al. Attributable mortality of ICU-acquired bloodstream infections: impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect. 2017;74(2):131–41. https://doi.org/10.1016/j. jinf.2016.11.001. Medline:27838521
5. Canadian Nosocomial Infection Surveillance Program. CNISP HAI surveillance case definitions. 2018. https://ipac-canada.org/photos/custom/Members/ CNISP publications/2018_CNISP_HAI%20 Surveillance_Case_Definitions_EN.pdf (Accessed December 6, 2021). 6. National Healthcare Safety Network. Ventilator-associated event (VAE). 2020. https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf (Accessed October 2020) 7. Leffler DA, Lamont JT. Clostridium difficile infection. New Eng J Med. 2015 Apr 16;372(16):1539–48. https:// doi.org/10.1056/NEJMra1403772. Medline:25875259
8. Mulvey MR, Simor AE. Antimicrobial resistance in hospitals: how concerned should we be? CMAJ. 2009;180(4):408–15. https://doi.org/10.1503/ cmaj.080239. Medline:19221354
9. Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am. 2016;42(1):157–76. https://doi. org/10.1016/j.rdc.2015.08.004. Medline:26611557
10. Boxer LA. How to approach neutropenia. Hematology Am Soc Hematol Educ Program. 2012;2012: 174–82. https://doi.org/10.1182/asheducation. V2012.1.174.3798251. Medline:23233578
11. Topster.net. Hours calculator (How many hours). https://www.topster.net/calendar/stundenrechner.php (Accessed December 2019).
12. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–111. https://doi.org/10.1093/cid/ ciw353. Medline:27418577
13. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67. https://doi.org/10.1164/ rccm.201908-1581ST. Medline:31573350
14. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388. https://doi. org/10.1164/rccm.200405-644ST. Medline:15699079
15. Seppala H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. New Eng J Med. 1997;337(7):441–6. https://doi.org/10.1056/ NEJM199708143370701. Medline:9250845
16. Hyde TB, Gay K, Stephens DS et al. Macrolide resistance among invasive Streptococcus pneumoniae isolates. JAMA. 2001;286(15):1857–62. https://doi. org/10.1001/jama.286.15.1857. Medline:11597287
17. Chen DK, McGeer A, de Azavedo JC, Low DE. Decreased susceptibility of Streptococcus pneu-moniae to fluoroquinolones in Canada. New Eng J Med. 1999;341(4):233–9. https://doi.org/10.1056/ NEJM199907223410403. Medline:10413735
18. Rhee C, Kadri SS, Dekker JP, et al. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3(4):e202899. https://doi.org/10.1001/jamanet-workopen.2020.2899. Medline:32297949
19. Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9. https://doi.org/10.1001/jama.2009.1754. Medline:19952319
20. Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System – report 2016. https:// www.canada.ca/content/dam/phac-aspc/documents/ services/publications/drugs-health-products/antibiotic-resistance-antibiotique/antibiotic-resistance-antibiotique- 2016-eng.pdf (Accessed August 2020).



