Reprinted with permission from the Official Journal of the Association of Medical Microbiology and Infectious Disease Canada.
Oyungerel Byambasuren MD1, Magnolia Cardona PhD1, Katy Bell PhD2, Justin Clark BA1, Mary-Louise McLaws PhD3, Paul Glasziou PhD1
1Institute for Evidence-Based Healthcare, Bond University, Gold Coast, Queensland, Australia
2School of Public Health, University of Sydney, Sydney, New South Wales, Australia
3School of Public Health and Community Medicine, UNSW Sydney, Sydney, New South Wales, Australia
Corresponding author:
Oyungerel Byambasuren, Institute for Evidence-Based Healthcare, Bond University, 14 University Drive, Robina, Gold Coast, Queensland 4229, Australia
obyambas@bond.edu.au
ABSTRACT
Background: Knowing the prevalence of true asymptomatic coronavirus disease 2019 (COVID-19) cases is critical for designing mitigation measures against the pandemic. We aimed to synthesize all available research on asymptomatic cases and transmission rates.
Methods: We searched PubMed, Embase, Cochrane COVID-19 trials, and Europe PMC for primary studies on asymptomatic prevalence in which
(1) the sample frame includes at-risk populations, and;
(2) follow-up was sufficient to identify pre-symptomatic cases. Meta-analysis used fixed-effects and random-effects models. We assessed risk of bias by combination of questions adapted from risk of bias tools for prevalence and diagnostic accuracy studies.
Results: We screened 2,454 articles and included 13 low risk-of-bias studies from seven countries that tested 21,708 at-risk people, of which 663 were positive and 111 asymptomatic. Diagnosis in all studies was confirmed using a real-time reverse transcriptase–polymerase chain reaction test. The asymptomatic proportion ranged from 4% to 41%. Meta-analysis (fixed effects) found that the proportion of asymptomatic cases was 17% (95% CI 14% to 20%) overall and higher in aged care (20%; 95% CI 14% to 27%) than in non-aged care (16%; 95% CI 13% to 20%). The relative risk (RR) of asymptomatic transmission was 42% lower than that for symptomatic transmission (combined RR 0.58; 95% CI 0.34 to 0.99, p = 0.047).
Conclusions: Our one-in-six estimate of the prevalence of asymptomatic COVID-19 cases and asymptomatic transmission rates is lower than those of many highly publicized studies but still sufficient to warrant policy attention. Further robust epidemiological evidence is urgently needed, including in subpopulations such as children, to better understand how asymptomatic cases contribute to the pandemic.
KEYWORDS
Emerging or re-emerging diseases, epidemiology, evidence-based medicine, public health policy
RÉSUMÉ
Historique : Il est essentiel de connaître la prévalence des véritables cas asymptomatiques de maladie à coronavirus 2019 (COVID-19) pour concevoir des mesures d’atténuation de la pandémie. Les chercheurs ont voulu synthétiser toutes les recherches disponibles sur les cas asymptomatiques et les taux de transmission.
Acknowledgments: The authors thank the authors of eligible articles for their replies to our queries.
Contributors: Conceptualization, OB, MC, KB, PG; Methodology, OB, MC, KB, JC, M-LM, PG; Software, JC; Formal Analysis, OB, MC, KB, PG; Data Curation, MC, KB, M-LM, PG; Writing – Original Draft, OB, MC, KB, JC, M-LM, PG; Writing – Review & Editing, OB, MC, KB, JC, M-LM, PG; Visualization, OB; Supervision, PG; Project Administration, OB.
Funding: OB is supported by National Health and Medical Research Council (NHMRC) grant APP1106452. PG is supported by NHMRC Australian Fellowship grant 1080042. KB is supported by NHMRC Investigator grant 1174523. All authors had full access to all data and agreed on the final manuscript submitted for publication. This study had no funding source.
Disclosures: Mary-Louise McLaws is a member of the World Health Organization (WHO) Health Emergencies Program Experts Advisory Panel for Infection Prevention and Control (IPC) Preparedness, Readiness and Response to COVID-19 and WHO IPC Guidance Development Group for COVID-19.
Méthodologie : Les chercheurs ont fouillé les bases de données PubMed, Embase, Cochrane pour trouver les études sur la COVID-19, et Europe PMC pour colliger les études primaires sur la prévalence des cas asymptomatiques dans lesquelles 1) le cadre d’échantillonnage incluait une population à risque et 2) le suivi était suffisant pour dépister les cas présymptomatiques. La méta-analyse a fait appel à des modèles d’effets fixes et d’effets aléatoires. Nous avons évalué le risque de biais par une combinaison de questions adaptées d’outils sur les risques de biais des études de prévalence et de précision diagnostique.
Résultats : Les chercheurs ont extrait 2 454 articles, dont 13 études à faible risque de biais de sept pays dans lesquelles 21 708 personnes à risque ont subi le test de dépistage, soit 663 cas positifs et 111 cas asymptomatiques. Dans toutes les études, le diagnostic a été confirmé au moyen du test d’amplification en chaîne par polymérase après transcriptase inverse en temps réel. La proportion de cas asymptomatiques se situait entre 4 % et 41 %. La méta-analyse (à effets fixes) a établi que la proportion de cas asymptomatiques s’élevait à 17 % (IC à 95 %, 14 % à 20 %) dans l’ensemble, mais qu’elles étaient plus élevées dans les soins aux aînés (20 %; IC à 95 %, 14 % à 27 %) qu’auprès du reste de la population (16 %; IC à 95 %, 13 % à 20 %). Le risque relatif [RR] de transmission de cas asymptomatiques était plus faible de 42 % que celui de cas symptomatiques (RR combiné de 0,58; IC à 95 %, 0,34 à 0.99, p = 0,047).
Conclusions : L’évaluation de la prévalence d’un sixième de cas asymptomatiques de COVID-19 et de taux de transmission de cas asymptomatiques est inférieure à celle de nombreuses études hautement publicisées, mais suffit tout de même pour justifier l’intérêt de la santé publique. D’autres données épidémiologiques solides s’imposent de toute urgence, y compris dans des sous-populations comme les enfants, pour mieux comprendre l’effet des cas asymptomatiques sur la pandémie.
MOTS-CLÉS
Epidémiologie, maladie émergente ou réémergente, médecine fondée sur des données probantes, politique de santé publique
INTRODUCTION
Asymptomatic cases of any infection are of considerable concern for public health policies to manage epidemics. Such asymptomatic cases complicate the tracking of an epidemic and prevent reliable estimates of transmission, tracing, and tracking strategies for containing an epidemic through isolation and quarantine. This has been a significant concern in the current coronavirus disease 2019 (COVID-19) pandemic [1].
The possibility of asymptomatic transmission of COVID-19 cases was first raised by a case report in China in which a traveller from Wuhan was presumed to have transmitted the infection to five other family members in other locations while she remained asymptomatic for the entire 21-day follow-up period [2]. Subsequently, other reports confirmed not only the possibility of such transmission but began quantifying the potential proportions. For example, the outbreak on the Diamond Princess cruise ship included a substantial proportion of asymptomatic cases after widespread testing of those on board the ship [3]. An early rapid review by the Centre for Evidence-Based Medicine in Oxford, United Kingdom, found that the estimated proportion of asymptomatic COVID-19 cases ranged from 5% to 80% [4]. However, many of the identified studies were either poorly executed or poorly documented, making the validity of these estimates questionable.
We therefore sought to identify all studies that had attempted to estimate the proportion of asymptomatic COVID-19 cases, select those with low risk of bias, and synthesize them to provide an overall estimate and potential range. We also aimed to estimate the rate of forward transmission from asymptomatic cases if sufficient data were found.
METHODS
We conducted a systematic review and meta-analysis using enhanced processes with an initial report completed within two weeks and daily short team meetings to review progress, plan the next actions, and resolve discrepancies and other obstacles [5]. We also used locally developed open access automation tools and programs such as the Polyglot Search Translator, SearchRefiner, and the SRA Helper to design, refine, and convert our search strategy for all the databases we searched and to speed up the screening process [6]. We searched the PROSPERO database to rule out the existence of a similar review and PubMed, Embase, and Cochrane COVID-19 trials for published studies and Europe PMC for pre-prints from January 2020 to July 20, 2020. A search string composed of MeSH terms and words was developed in PubMed and was translated to be run in other databases using the Polyglot Search Translator. The search strategies for all databases are presented in Supplemental Appendix 1. We also conducted forward and backward citation searches of the included studies in the Scopus citation database.
We restricted publication types to reports of primary data collection released in full (including pre-prints) with sufficient details to enable a risk-of-bias assessment, and we contacted authors for clarifications on follow-up
times and sampling frames. We anticipated that cross-sectional prevalence surveys with follow-up and cohort studies would be the bulk of eligible reports. No restrictions on language were imposed. We excluded studies for the following reasons: sampling frame in part determined by presence or absence of symptoms; no or unclear follow-up; no data on asymptomatic cases; single case study or small cluster; modelling or simulation studies (but sources of real data were checked for possible inclusion); non–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) studies; antiviral treatment studies; and study protocols, guidelines, editorials, or historical accounts without data to calculate primary outcomes.
Participants
We included studies of people of any age in which all those at risk of contracting SARS-CoV-2 were tested regardless of presence or absence of symptoms; diagnosis was confirmed by a positive result on a real-time reverse transcriptase–polymerase chain reaction (RT-PCR), and all cases had a follow-up period of at least seven days to distinguish asymptomatic cases from pre-symptomatic cases (Figure 1).
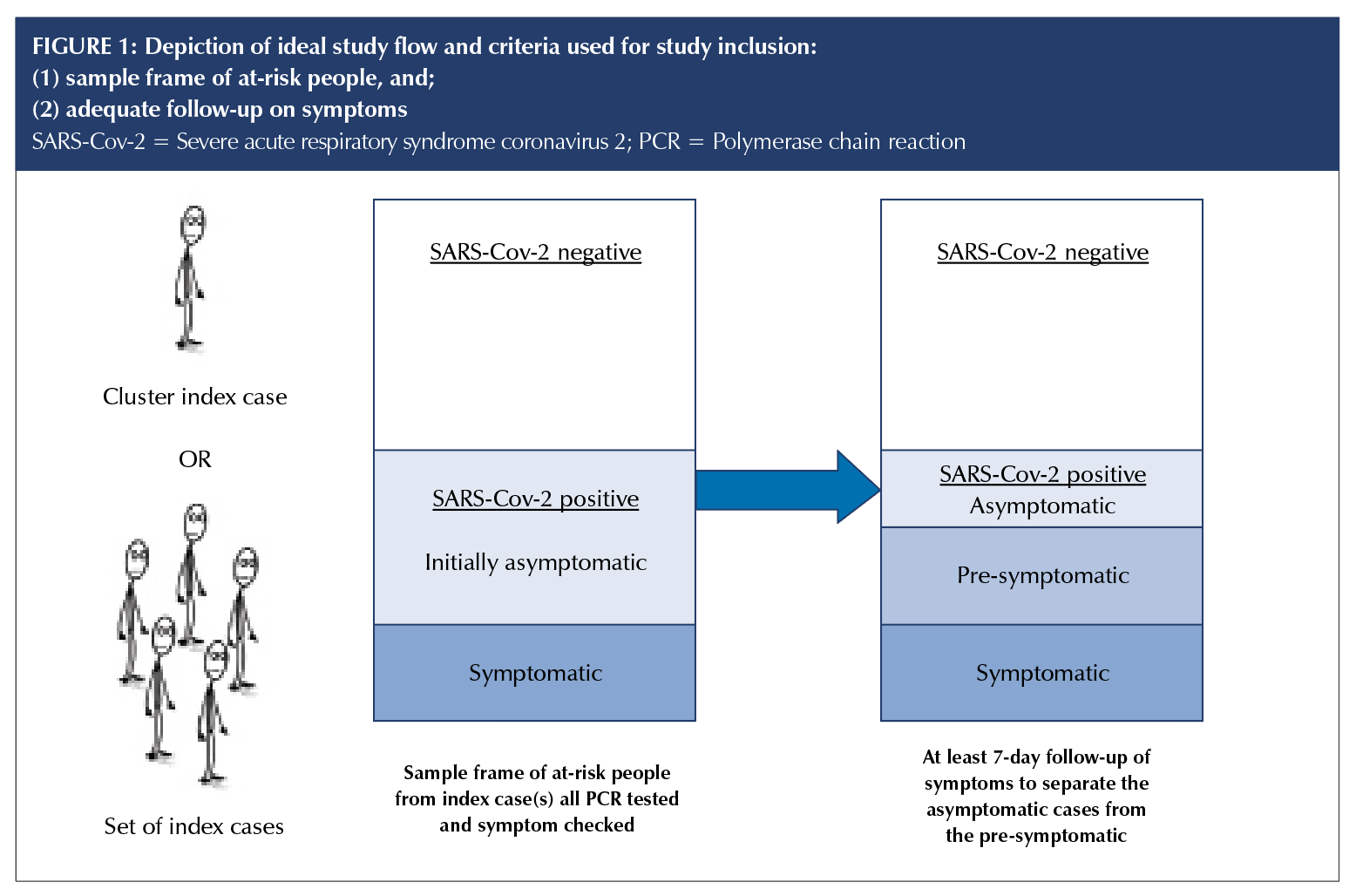
Outcomes
Our primary outcome was the proportion of all people with SARS-Cov-2 infection who were completely asymptomatic at the time of the test and throughout the follow-up period, where the denominator included all tested individuals in the study sample whose result was positive, and the numerator included those who tested positive and had no symptoms. Our secondary outcome was estimate of onward transmission of the infection from asymptomatic cases.
Study selection and screening
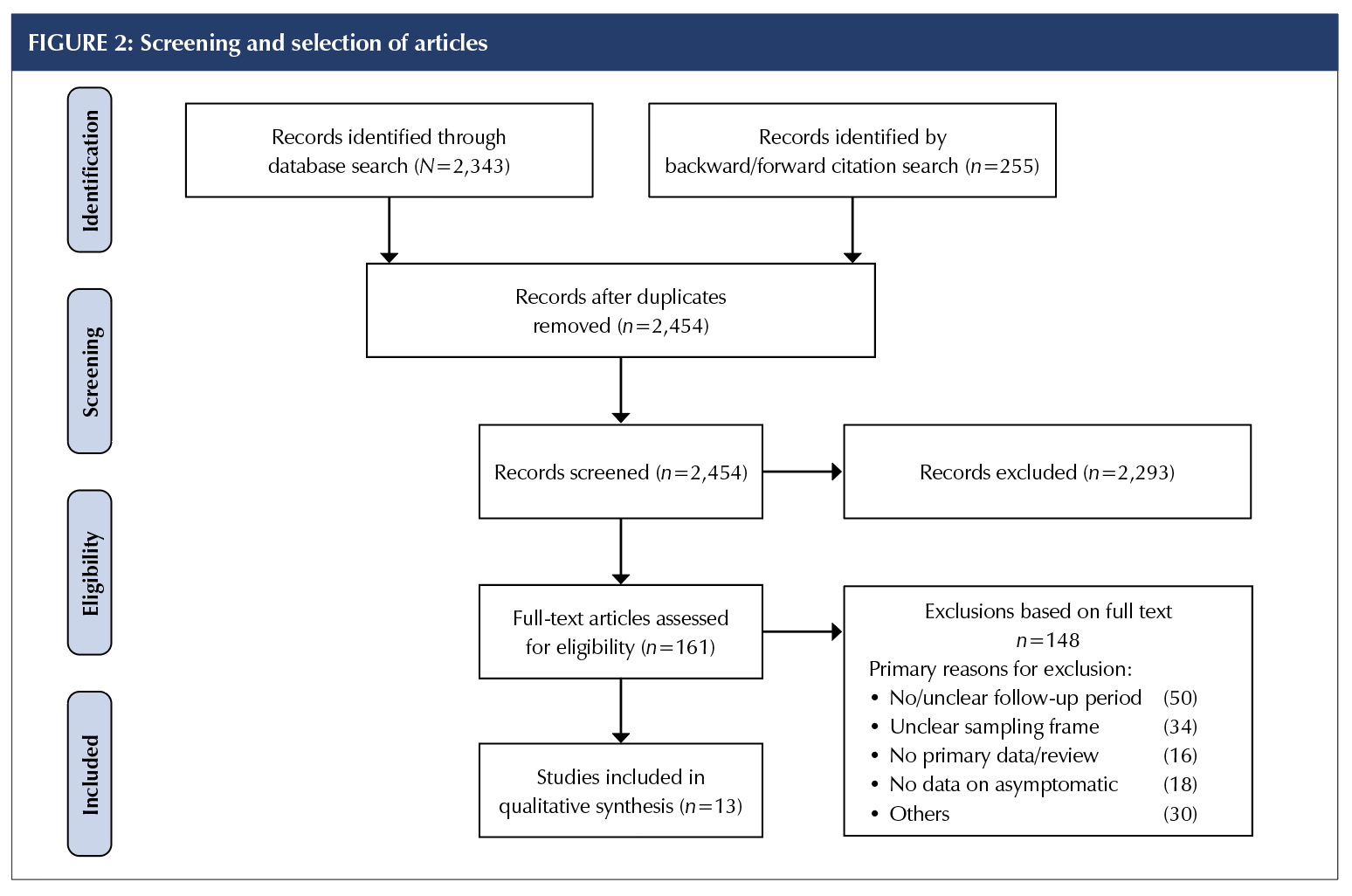
Two authors (OB and MC) independently screened titles, abstracts, and full texts according to eligibility criteria.
All discrepancies were resolved via group discussion with
the other authors. Reasons for exclusion were documented
for all full-text articles deemed ineligible (Supplemental Appendix 2); see the Preferred Reporting Information for Systematic Reviews and Meta-Analyses diagram (Figure 2).
Data extraction
Three authors (OB, MC, KB) used a Microsoft Excel spreadsheet to extract the following information:
- Methods: study authors, year of publication, country, publication type, duration of study, duration of follow-up
- Participants: sample size, age (mean or median, range), setting (community, province, aged care facility, hospital, screening clinic), presence or absence of symptoms, test results
- History of illness and diagnosis: type of test; numerator (number of asymptomatic); denominator (sampling frame); mildly symptomatic or symptomatic subjects; and number or proportion of people infected by the asymptomatic case.
- Case definitions were as follows:
-
- Asymptomatic: confirmed via any testing specified earlier with report of no symptoms for the duration of sufficient follow-up to differentiate from pre-symptomatic cases.
- Exposure: contact with a confirmed case or potential contact with another pre-symptomatic person
(e.g., came from an endemic area or linked with an infected traveller).
The World Health Organization recommends that “for confirmed asymptomatic cases, the period of contact is measured as the two days before through the 14 days after the date on which the sample was taken which led to confirmation” (7, p.11).
Risk-of-bias assessment
Three authors (OB, MC, KB) assessed the risk of bias of potentially includable studies. We used a combination of risk-of-bias tools for prevalence studies and diagnostic accuracy and adapted the key signaling questions on sampling frame, ascertainment of infectious disease status, acceptability of methods to identify denominators, case definition of asymptomatic for the numerator, and length of follow-up, as shown in Table 2 and in Supplemental Appendix 3 in full [8,9].
Data analysis
We estimated the proportion of COVID-19 cases who were asymptomatic for each included study population, assuming a binomial distribution and calculating exact Clopper–Pearson confidence intervals. We then pooled data from all included studies using:
(1) fixed-effects meta-analysis and
(2) random-effects meta-analysis. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC); the FREQ procedure was used for individual studies
and the fixed-effects meta-analysis; the NLMIXED procedure was used for the random-effects meta-analysis. We also meta-analyzed the forward transmission rates from asymptomatic and symptomatic cases when there were sufficient data and report the pooled RR comparing the two. We planned to undertake subgroup analysis for age (between studies, and within studies when age was reported separately for asymptomatic and symptomatic cases). Because the analysis included only studies deemed to be of high quality on items 1 and 2 after risk-of-bias appraisal, no sensitivity analysis of high- versus low-quality studies was undertaken. Instead, we did a sensitivity analysis in which we omitted studies with a follow-up duration of less than 14 days.
RESULTS
A total of 2,454 articles were screened for title and abstract, and 161 full-text articles were assessed for inclusion (Figure 2). Major reasons for exclusion were inadequate sampling frame and insufficient follow-up time to accurately classify the asymptomatic cases. The full list of excluded studies with reasons is presented in Supplemental Appendix 2. Thirteen articles – nine published and four preprints – from seven countries (China, n = 4; United States, n = 4; Taiwan, n = 1; Brunei, n = 1; Korea, n = 1; France, n = 1; and Italy, n = 1) that tested 21,708 close contacts of at least 849 confirmed COVID-19 cases, of which 663 were positive and 111 were asymptomatic, met the eligibility criteria for the estimation of the primary outcome [10–22].The sampling frames of the selected studies included residents of skilled nursing facilities (SNFs; 10, 12, 15, 19, 20); high-risk close contacts of confirmed COVID-19 cases [11, 13, 14, 17, 18, 21]; and a whole district surveillance program in Italy [16]. The demographic characteristics (Table 1) indicate that most of the tested individuals were adults, with a mean age of more than 75 years in the five SNF studies and a mean age of more than 31 years in the non-aged care studies. The proportions of children and young people (0-20 years) ranged from 6% to 23.5%.
Diagnosis in all studies was confirmed via RT-PCR and in two cases was supplemented with radiological evidence [17, 21]. Testing of individuals in the study sample varied across settings but was generally very high: all contacts regardless of symptoms [11, 13, 14, 17, 18, 21], more than 97% of SNF residents [10, 12, 15, 19, 20], and 85.9% of an entire town [16]. The length of follow-up for monitored individuals in the SNF studies ranged from seven to 30 days [10, 12, 15, 19, 20]; 14 days for the Bruneian [13], Taiwanese [14], Korean [18], and Chinese close contacts [17, 22]; seven to 14 days in the Italian community [16]; 12 days for 95% of all contacts in the Shenzhen community surveillance [11]; and a mean of 16 (SD 6) days in Liaocheng, China [21].
The proportion of asymptomatic cases in the 13 included studies ranged from 4% (95% CI 1% to 10%) in Korea [18] to 40% in Vò, Italy [16] and in an aged care facility in the United States [20]. Combining data from all 13 studies, we estimate that 17% of cases were asymptomatic (fixed effects 95% CI 14% to 20%;); for the eight non-aged care studies, 16% (95% CI 13% to 19%); and for the five studies of SNFs, 20% (95% CI 14% to 27%) (Figure 3). The corresponding estimated proportions in the random-effects meta-analysis were, overall, 18% (95% CI 9% to 26%); non-aged care, 16% (95% CI 7% to 26%); and aged care, 21% (95% CI 5% to 36%). The 95% prediction interval was 4% to 52%. In the sensitivity analysis, which omitted studies in which length of follow-up was less than 14 days [10, 11, 16, 20], the overall estimate was modestly lower at 15% (fixed-effects 95% CI 12% to 18%) or 17% (random-effects 95% CI 8 to 26%). Heterogeneity as expressed by I2 was 84%.
Five studies reported data on secondary infection transmission from asymptomatic cases (Table 2). The asymptomatic transmission rates ranged from none to 2.2%, whereas symptomatic transmission rates ranged between 0.8% and 15.4%. Cycle threshold from real-time RT-PCR assays or the viral load did not differ between asymptomatic and symptomatic individuals in three of the studies [10, 14, 16]. Overall, the RR of asymptomatic transmission was 42% lower than that of symptomatic transmission (pooled RR 0.58, fixed-effects 95% CI 0.335 to 0.994, p = 0.047; RR 0.38, random-effects 95% CI 0.13 to 1.083, p = 0.07; I2 = 43.4%).
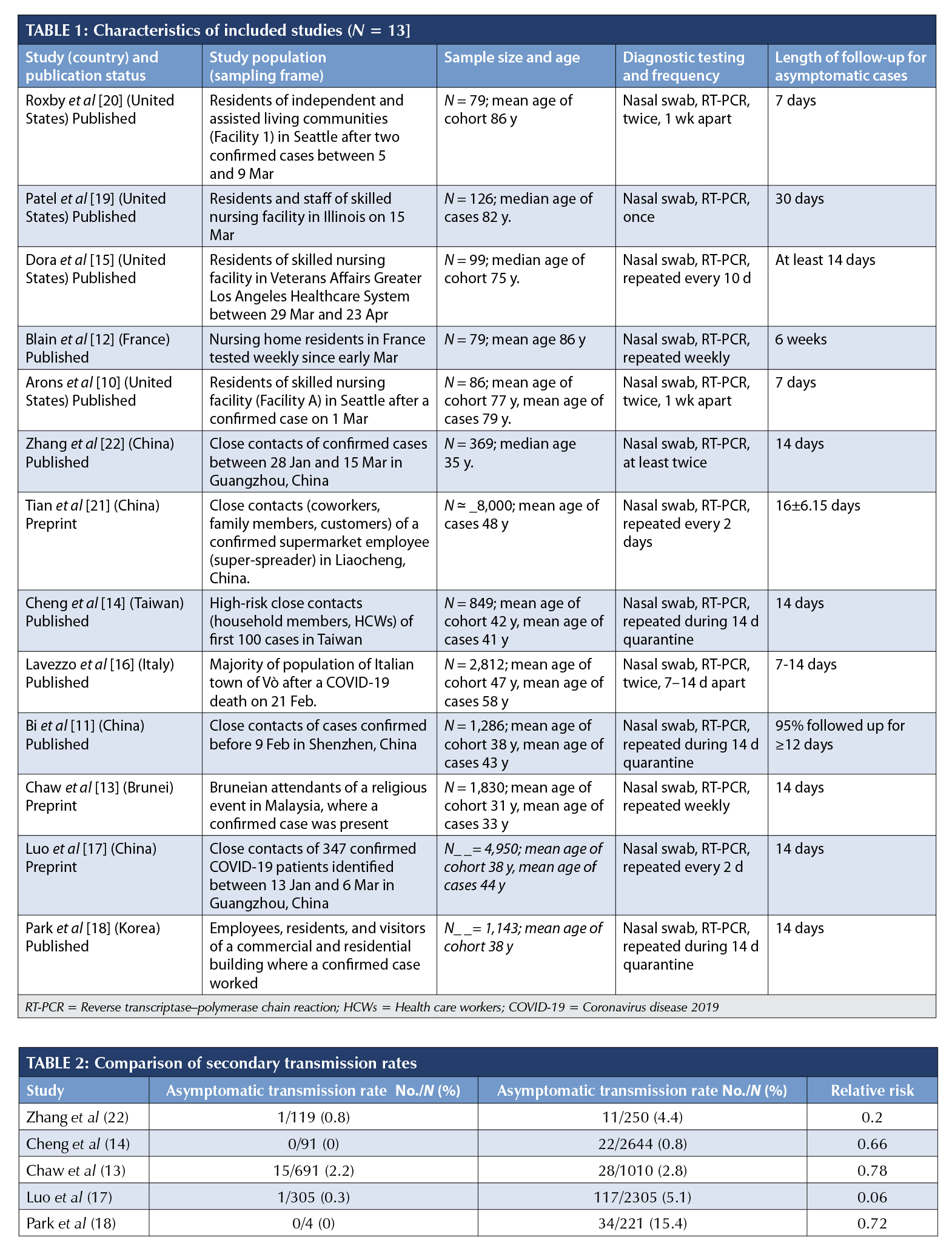
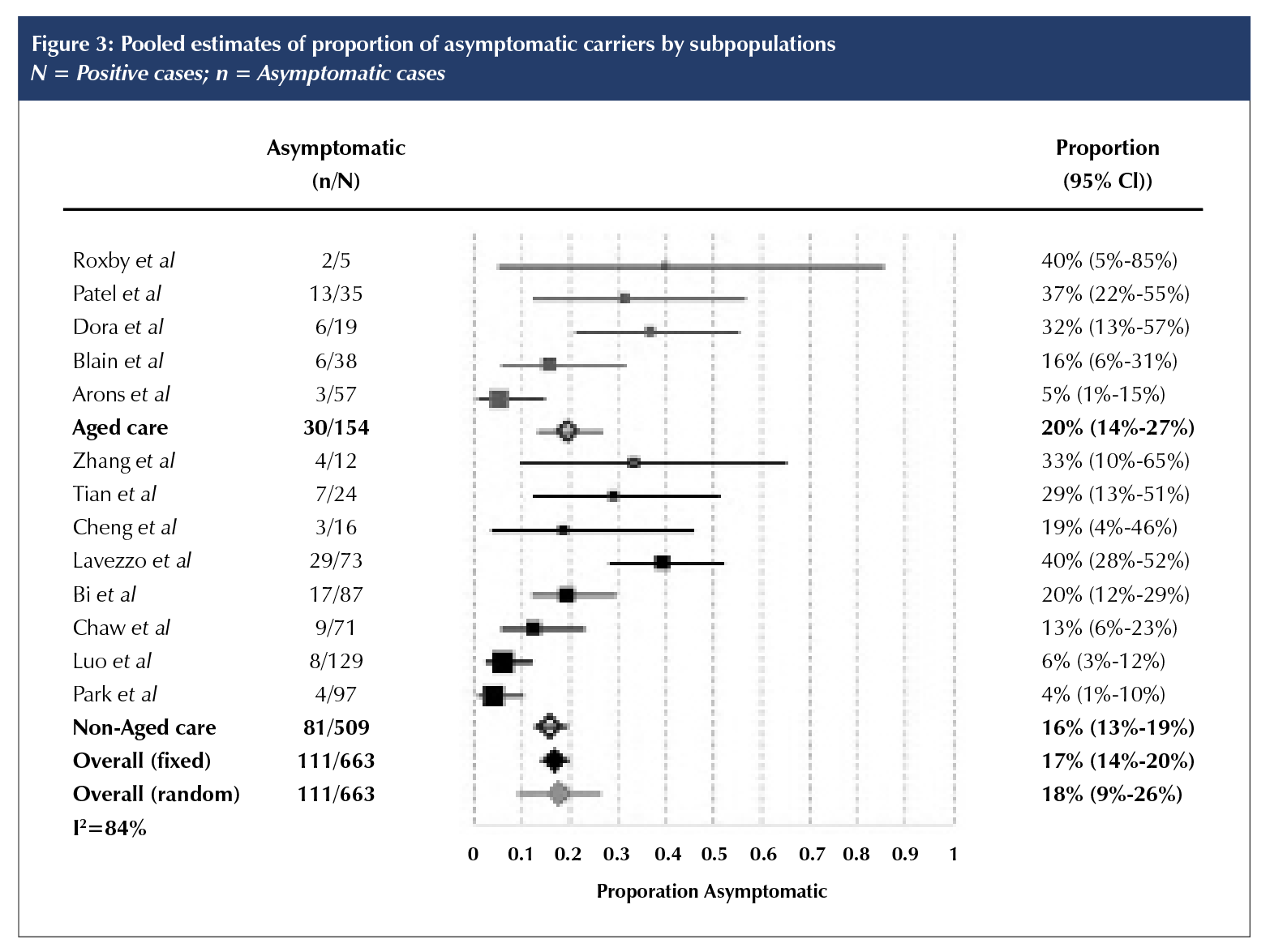

Risk of bias of included studies
Table 3 summarizes the overall risk-of-bias assessment of the nine included studies (the full list of risk-of-bias questions is in Supplemental Appendix 3). All of the studies were evaluated as low risk of bias for the sampling frame and length of follow-up domains (domains 1 and 5), which were part of the inclusion criteria. Two studies had potential non-response bias because not all of the eligible participants were tested (14% [463/3,275] of the target population was not tested in the Lavezzo et al study [16] or results were not reported for all tested participants (87/98 cases were reported in the Bi et al study [11]; domain 2). Four studies either had not tested the study population at least twice during the follow-up period or had not provided clear information on testing [11, 13, 14, 21] (domain 3). Nine studies did not explicitly state the asymptomatic case definition they adhered to or had additional bias because of a high percentage of people in the SNFs with severe cognitive impairment [10–12, 14–16, 19-21] (domain 4).
Excluded studies
Several well-publicized studies did not meet our inclusion criteria. The outbreak on the Diamond Princess cruise ship involved 3,711 passengers, of whom more than 600 acquired COVID-19 [3]. Many of the positive cases were relocated to medical facilities in Japan without details of their clinical progression. To correct for the lack of follow-up, Mizumoto et al applied a statistical adjustment for the right censoring and estimated that 17.9% (95% CI 15.5% to 20.2%) of positive cases were asymptomatic.
An open-invitation screening of the Icelandic population suggested that around 0.8% of the population were SARS-CoV-2 positive, with half classified as (initially) asymptomatic [2]. However, because there was no follow-up, we cannot separate asymptomatic from pre-symptomatic individuals. Moreover, the study excluded symptomatic people undergoing targeted testing, which impeded estimation of an overall asymptomatic rate.
A study of 215 pregnant women in New York identified 33 SARS-CoV-2–positive women [23]. On admission to the delivery unit, four of the 33 positive cases were symptomatic and three became symptomatic before postpartum discharge, suggesting an asymptomatic rate of 79% (26/33). However, the two days of follow-up were insufficient to meet our inclusion criteria.
A case report of a pre-symptomatic Chinese businessman transmitting COVID-19 to a German business partner was also excluded because despite three other people acquiring the infection from the infected German source, none of them was asymptomatic at follow-up [24]. A five-day point-prevalence testing of adults living in homeless shelters in Boston found 147 positive cases, of which the majority had mild or no symptoms [25]. We excluded this study because no numeric estimate was included of those who were truly asymptomatic, and there was no follow-up assessment.
Two studies examined people repatriated from overseas to their home countries by plane. Neither study was clear on whether symptomatic people could board the plane and be included, and if they were excluded, the asymptomatic rates would be overestimated. A study of 565 Japanese citizens repatriated from China [26] found 13 positives – four asymptomatic and nine symptomatic, based on screening on arrival. Another study of 383 Greek citizens repatriated from the United Kingdom, Spain, and Turkey [27] found 40 asymptomatic positive people on arrival, four of whom later self-reported symptoms. Again, the likely initial exclusion of symptomatic people and the lack of comprehensive follow-up would both result in overestimation of the asymptomatic rates.
DISCUSSION
Principal findings
Although the rate of asymptomatic COVID-19 cases has received considerable attention, we found only 13 studies that provided an adequate sample frame and follow-up to ascertain a valid estimate of the proportion of asymptomatic cases. The combined estimate of the asymptomatic proportion was 17% (95% CI 14% to 20%) but had considerable heterogeneity (I2 = 84%) and
a 95% prediction interval that ranged from 4% to 52%.
There was no clear difference in the proportions between aged care and non-aged care studies. Only five of the 13 studies provided data on transmission rates from asymptomatic cases. The transmission risk from asymptomatic cases appeared to be lower than that of symptomatic cases, but there was considerable uncertainty in the extent of this (RR 0.58; 95% CI 0.335 to 0.994, p = 0.047).
Strengths and weaknesses of the study
Strengths of our systematic review include achieving full methodological rigor within a much shorter time frame than traditional reviews using enhanced processes and automation tools [5]. We also critically assessed the risk of bias of all full-text articles we screened to include studies with the least risk of bias in sampling frame and length of follow-up domains to be able to differentiate between asymptomatic and pre-symptomatic cases.
Our findings have several limitations. First, our search focused on published and pre-print articles, and we may have missed some public health reports that are either unpublished or only available on organizational websites. Second, the design and reporting of most of the studies had a number of important deficits that could affect their inclusion or our estimates. These deficits include poor reporting of the sample frame, testing and symptom check, and follow-up processes. Such reporting would have been considerably aided by including a flow chart of cases (as Lavezzo et al [16] did) with identification, testing, and follow-up, including missing data. A further important limitation was the poor reporting of symptoms, which was often simply dichotomized into symptomatic versus asymptomatic without clear definitions and details of possible mild symptoms. The included studies did not report sufficient data to examine the impact of age and underlying comorbidities on the asymptomatic rate. Finally, all included studies relied on RT-qPCR; hence, some cases might have been missed because of false-negative results, especially when study participants were only tested once [28]. If the tests missed more asymptomatic cases, then the true proportion of asymptomatic cases could be higher than our estimates. However, false-positive results, which may occur when people without symptoms are tested in low-prevalence settings, would mean the true prevalence of asymptomatic cases was lower than our estimates.
Strengths and weaknesses compared with other studies
Several other non-systematic and systematic reviews have examined the proportion of asymptomatic cases. The non-systematic reviews estimated asymptomatic rates as between 5% and 80% [4, 29]. However, they included only early cross-sectional reports and did not critically appraise the study design, nor did they attempt to pool the most valid studies. Five other systematic reviews reported pooled estimates of asymptomatic rate as between 8% and 16% [30–34]. However, these reviews included studies that we excluded because of high risk of bias in the sampling frame. Ongoing monitoring for new studies is warranted but should include robust methodological assessment, including ensuring included studies have a sufficient follow-up period to differentiate the asymptomatic from the pre-symptomatic cases. Our review currently also has a more recent search date than other reviews and includes sensitivity analysis by length of follow-up time. Our estimate of risk of transmission by asymptomatic cases was comparable to those reported in two other empirical reviews by Buitrago-Garcia et al (RR 0.35) and Koh et al (RR 0.39) [32, 34].
Meaning of the study
Estimates of the proportion of the cases that are asymptomatic and the risk of transmission are vital parameters for modelling studies. Our estimates of the proportion of asymptomatic cases and their risk of transmission suggest that asymptomatic spread is unlikely to be a major driver of clusters or community transmission of infection, but the extent of transmission risk for pre-symptomatic and minor symptomatic cases remains unknown. The generalisability of the overall estimate is unclear, and we observed considerable variation across the included studies, which had different settings, countries, and study design, reflected in the reasonably wide prediction interval.
Unanswered questions and future research
Many unanswered questions about asymptomatic cases remain. Only one of the more recent studies we included tested patients for immunoglobulin G antibodies to determine seroconversion among elderly individuals. Without repeated and widespread RT-PCR and antibody tests, it is difficult to accurately estimate the prevalence of COVID-19 infection and inform our infection prevention strategies [35]. The role of viral load and virus shedding dynamics in asymptomatic and symptomatic cases will further help answer the question of forward transmission and disease length and severity. Other unknowns include whether there is a difference in the proportion of cases that are asymptomatic according to age (particularly children versus adults), sex, or underlying comorbidities, and whether asymptomatic cases develop long-term immunity to new infections. For most studies, the PCR (positive) cases were traced from the index cases, and the testing was carried out mostly at the beginning of the pandemic wave for the locale. So, for this review of inception cohorts, people with long-term persistent positive testing were unlikely to be misclassified as asymptomatic. The issue of persistent PCR positivity after a person has recovered from infection might be of concern to more recent studies conducted at some time after the first wave of the pandemic. In such studies, researchers will need to ask about history of illness compatible with COVID-19 even if this occurred months ago, and PCR testing could be supplemented by other tests such as viral culture and anti-SARS-CoV-2 antibody tests.
Our recommendations for future research also include improved clearer reporting of methods, sampling frames, case definition of asymptomatic, extent of contact tracing, duration of follow-up periods, presentation of age distribution of asymptomatic cases, and separation of pre-symptomatic and mild cases from asymptomatic cases in results tables. Most studies used a limited definition of asymptomatic COVID-19 case, which could lead to mixing paucisymptomatic cases with asymptomatic cases. If that were a common issue, then the true prevalence of asymptomatic cases would be even lower than the current estimates. A reliable estimate of the proportion of asymptomatic cases and the burden of disease is imperative in understanding the infection transmission capacity of asymptomatic cases to inform public health measures for these individuals who, according to our findings, appear to pose lower risk of transmission. Until we have further immunological and epidemiological evidence, we advise that the importance of asymptomatic cases for driving the spread of pandemic to be considered with caution.
REFERENCES
1. Yuen K-S, Ye ZW, Fung S-Y, Chan C-P, Jin D-Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Bioscience. 2020;10(1):40. https://doi.org/10.1186/s13578-020-00404-4. Medline: 32190290
2. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–7. https://doi.org/10.1001/jama.2020.2565. Medline: 32083643
3. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10). https://doi.org/10.2807/1560-7917.ES.2020.25.10.2000180. Medline: 32183930
4. Heneghan C, Brassey J, Jefferson T. COVID-19: what proportion are asymptomatic? Centre for Evidence-Based Medicine, Oxford, UK. 2020. https://www.cebm.net/covid-19/covid-19-what-proportion-are-asymptomatic/ (Accessed April 5, 2020).
5. Clark J, Glasziou P, Del Mar C, Bannach-Brown A, Stehlik P, Scott AM. A full systematic review was completed in two weeks using automation tools: a case study. J Clin Epidemiol. 2020;121:81–90. https://doi.org/10.1016/j.jclinepi.2020.01.008. Medline: 32004673
6. Clark JM, Sanders S, Carter M, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc. 2020;108(2):195–207. https://doi.org/10.5195/jmla.2020.834. Medline: 32256231
7. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report–66. 2020 26 March; Geneva, Switzerland.
8. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–9. https://doi.org/10.1016/j.jclinepi.2011.11.014. Medline: 22742910
9. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009. Medline: 22007046
10. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020; 382:2081–90. https://doi.org/10.1056/NEJMoa2008457. Medline: 32329971
11. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infectious Diseases. 2020; 20(8):P911–9. https://doi.org/10.1016/S1473-3099(20)30287-5.
12. Blain H, Rolland Y, Tuaillon E, et al. Efficacy of a test-retest strategy in residents and health care personnel of a nursing home facing a COVID-19 outbreak. J Am Med Dir Assoc. 2020;21(7):933–6. https://doi.org/10.1016/j.jamda.2020.06.013. Medline: 32674822
13. Chaw L, Koh WC, Jamaludin SA, Naing L, Alikhan MF, Wong J. SARS-CoV-2 transmission in different settings: analysis of cases and close contacts from the Tablighi cluster in Brunei Darussalam. medRxiv; 2020. https://doi.org/10.1101/2020.05.04.20090043.
14. Cheng H-Y, Jian S-W, Liu D-P, Ng T-C, Huang W-T, Lin H-H. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020; 180(9):1156–63 https://doi.org/10.1001/jamainternmed.2020.2020. Medline: 32356867
15. Dora AV, Winnett A, Jatt LP, et al. Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for veterans–Los Angeles, California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(21):651–5. https://doi.org/10.15585/mmwr.mm6921e1. Medline: 32463809
16. Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vò. Nature. 2020; 584:425–9.
17. Luo L, Liu D, Liao X-l, et al. Modes of contact and risk of transmission in COVID-19 among close contacts. medRxiv; 2020. https://doi.org/10.1101/2020.03.24.20042606.
18. Park SY, Kim YM, Yi S, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26(8):1666–70. https://doi.org/10.3201/eid2608.201274. Medline: 32324530
19. Patel MC, Chaisson LH, Borgetti S, et al. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis. 2020. (ePub Ahead of Print) https://doi.org/10.1093/cid/ciaa763. Medline: 32548628
20. Roxby AC, Greninger AL, Hatfield KM, et al. Detection of SARS-CoV-2 among residents and staff members of an independent and assisted living community for older adults–Seattle, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):416–8. https://doi.org/10.15585/mmwr.mm6914e2. Medline: 32271726
21. Tian S, Wu M, Chang Z, et al. Epidemiological investigation and intergenerational clinical characteristics of 24 COVID-19 patients associated with supermarket cluster. medRxiv; 2020. https://doi.org/10.1101/2020.04.11.20058891.
22. Zhang W, Cheng W, Luo L, et al. Secondary transmission of coronavirus disease from presymptomatic persons, China. Emerg Infect Dis. 2020;26(8). https://doi.org/10.3201/eid2608.201142. Medline: 32453686
23. Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery (Lett). New Engl J Med. 2020;382:2163–4 https://doi.org/10.1056/NEJMc2009316. Medline: 32283004
24. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–1.
https://doi.org/10.1056/NEJMc2001468. Medline: 32003551
25. Baggett TP, Keyes H, Sporn N, Gaeta JM. COVID-19 outbreak at a large homeless shelter in Boston: implications for universal testing. medRxiv. 2020. https://doi.org/10.1101/2020.04.12.20059618.
26. Nishiura H, Kobayashi T, Suzuki A, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) (Lett). Int J Infect Dis. 2020;9:P154–5. https://doi.org/10.1016/j.ijid.2020.03.020.
27. Lytras T, Dellis G, Flountzi A, et al. High prevalence of SARS-CoV-2 infection in repatriation flights to Greece from three European countries. J Travel Med. 2020;27(3):taaa054. https://doi.org/10.1093/jtm/taaa054. Medline: 32297940
28. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):200642. https://doi.org/10.1148/radiol.2020200642. Medline: 32101510
29. Oran D, Topol E. Prevalence of asymptomatic SARS-CoV-2 infection. Ann Intern Med. 2020;M20-3012. https://doi.org/10.7326/M20-3012. Medline: 32491919
30. Al-Sadeq DW, Nasrallah GK. The incidence of the novel coronavirus SARS-CoV-2 among asymptomatic patients: a systematic review. Int J Infect Dis. 2020;98:372–80 https://doi.org/10.1016/j.ijid.2020.06.098. Medline: 32623083
31. Beale S, Hayward A, Shallcross L, Aldridge R, Fragaszy E. A rapid review of the asymptomatic proportion of PCR-confirmed SARS-CoV-2 infections in community settings. medRxiv; 2020. https://doi.org/10.1101/2020.05.20.20108183.
32. Buitrago-Garcia DC, Egli-Gany D, Counotte MJ, et al. The role of asymptomatic SARS-CoV-2 infections: rapid living systematic review and meta-analysis. medRxiv. 2020. https://doi.org/10.1101/2020.04.25.20079103.
33. He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virology. 2020. (ePub Ahead of Print) https://doi.org/10.1002/jmv.26326. Medline: 32691881
34. Koh WC, Naing L, Rosledzana MA, et al. What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate, serial interval, and asymptomatic infection. medRxiv. 2020. https://doi.org/10.1101/2020.05.21.20108746.
35. Byambasuren O, Dobler CC, Bell K, et al. Estimating the seroprevalence of SARS-CoV-2 infections: systematic review. medRxiv. 2020. https://doi.org/10.1101/2020.07.13.20153163.


