Sameer S. Kassim MBBChBAO, MSc, DTHM&H, DipRCPath1,2, Jared Bullard MD, FRCPC2,3,4; and Allison McGeer MD, FRCPC5
1 Department of Family Medicine, Faculty of Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada
2 Department of Medical Microbiology & Infectious Diseases, Faculty of Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada
3 Department of Pediatrics & Child Health, University of Manitoba, Winnipeg, Manitoba, Canada
4 Cadham Provincial Laboratory, Winnipeg, Manitoba, Canada
5 Department of Microbiology, Sinai Health System, Toronto, Ontario, Canada
Corresponding author:
Dr. Sameer S. Kassim
Cadham Provincial Laboratory
750 William Avenue
Winnipeg, Manitoba
Canada, R3C3Y1,
Tel: 204-945-1306,
Fax: 204-786-4770
kassims@myumanitoba.ca
ABSTRACT
We retrospectively studied the epidemiology and preventative measures in adult and paediatric patients admitted to high-acuity units in Winnipeg, Manitoba. A total of 307 specimens were received, respiratory viruses were detected in 112 individual patient charts (39.4%). The aetiology varied by age-group with mixed viral and bacterial infection, or multiple viral infections noted in 33.3% and 39% in adults and paediatric patients, respectively. Respiratory syncytial virus (RSV) and rhinovirus were detected in 23% and 13% of paediatric patients, whereas influenza and parainfluenza were detected in 6.4% and 5.9% of adult patients, respectively. Influenza vaccination was noted in the charts of 7.8% of adults and 55.8% of children. In total, 34 (58%) paediatric patients and 11 (22%) adult patients had documented infection control orders, with 31 (92%) and four (36%) of these meeting regional guidelines, respectively. This study showed divergent viral aetiology based on age-group, as well as an opportunity to improve healthcare personnel knowledge and documentation of vaccination and infection control protocol.
KEYWORDS: Vaccine, prevention, infection control measures, respiratory virus, healthcare personnel, intensive care units.
INTRODUCTION
Viral respiratory tract infections (vRTI) are usually acute self-limited illnesses, but can cause severe disease in adults and children with underlying chronic illnesses, which may require admission to intensive care units (ICU) [1].
Influenza and other respiratory viral infections have guidelines for diagnosis, treatment, chemoprophylaxis, as well as vaccination and infection control protocols to reduce the risk of transmission in Canada and the United States. [3,4]. Current studies have focused on different facets of this clinical entity, however there are striking differences in the aetiology, clinical case definitions, and management of vRTIs in patients admitted to adult and paediatric ICUs [1]. For example, a study by Keledis et al., showed that the most common cause of viral pneumonia in adults is influenza virus type A and B, however, those who were immunocompromised were more likely to have viral pneumonias caused by respiratory syncytial virus (RSV), cytomegalovirus (CMV), herpes simplex virus (HSV), varicella-zoster virus (VZV), adenovirus and rarely measles. In contrast, rhinoviruses and coronaviruses circulate in the paediatric communities [2]. Furthermore, clinical case definitions were noted to vary in their sensitivity and specificity depending on the case definition applied to a vRTI and the population studied. A study by Jiang et al., showed that the case definitions such as Acute Respiratory Infection (ARI) demonstrated under calling the number of cases in hospitalized patients [5]. Finally, prior to the COVID-19 pandemic, limited evidence was available in the published literature describing preventative measures such as documentation, adherence rates and implementation of infection control practices in intensive care pertaining to viral respiratory tract infections.
Thus, the objective of this study was two-fold: 1. To describe the aetiology and applicability of clinical case definitions for vRTIs detected in adult and paediatric ICUs and 2. To describe the documentation and use of preventative measures, including vaccination and implementation of infection control protocols in the ICU.
METHODS
We retrospectively reviewed results of viral respiratory testing for patients admitted to ICUs at seven hospitals in Manitoba, Canada between October 1, 2016 to May 30, 2017. The hospitals included were two tertiary care facilities, one woman’s and paediatric hospital and four community hospitals representing 64 distributed critical-care beds, 10 of which were fully staffed beds in community centres in the adult program and 10 paediatric and 60 neonatal beds, in the paediatric and woman’s hospital, respectively.
Tests were requested by emergency providers, admitting critical care physicians, and/or infectious disease consultants based on clinical suspicion. Nasopharyngeal swabs, endotracheal swabs and/or bronchoalveolar lavage specimens were sent for analysis. All specimens were tested utilizing the qualitative SeeGene AllplexTM (RV16) assay, which detects: influenza A (H1, H1pdm09, H3), influenza B; respiratory syncytial virus A, B; adenovirus; enterovirus; parainfluenza virus (1-4); metapneumovirus; bocavirus and coronavirus (NL63, 229E, OC43).
Regional infection control protocols were derived for the adult and paediatric programs, respectively, and reflect best evidence-based practice guidance and standardized infection prevention and control practices across all facilities in the health region. The manuals are developed by the infection prevention and control committee and contain policies, operational directives, and protocols which are reviewed on an annual basis. These manuals are based on Accreditation Canada requirements, Public Health Agency of Canada recommendations and critical or practice changing literature. Infection control orders are documented either on specific infection control order sets, or on general orders if unavailable for all hospitals participating in this study.
Charts of patients with positive results were reviewed to identify risk factors, symptoms and signs of infection, antimicrobial usage, documentation of influenza vaccination, outcomes and application of regional infection control (IC) guidelines. Data was collected and entered into a secure excel spreadsheet by SSK; statistical analysis was performed using IBM SPSSv21. Differences were compared using X2 and Wilcoxon Rank sum tests. All p-values were two-sided with significant set at 0.05. This study was approved by the University of Manitoba-Research Ethics Board and the Health Information Privacy Committee – Government of Manitoba (2017/2018-55).
RESULTS
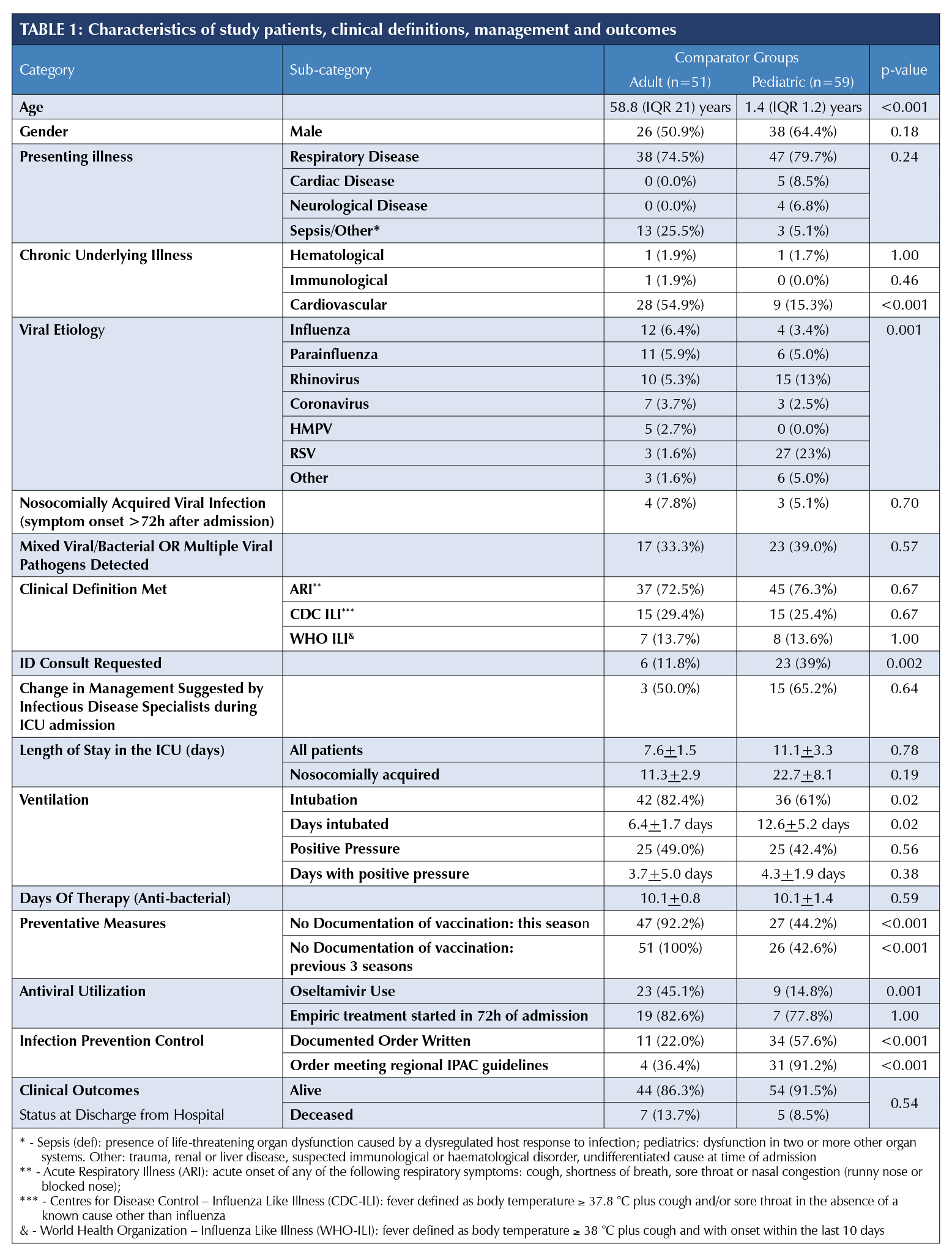
370 respiratory tract specimens (nasopharyngeal swabs/aspirates and bronchoalveolar lavage) were submitted for virus detection, 188 from adults (>=18 years), and 119 from children. At least one virus was detected in 112 individual patients (39.4%). 112 charts were available and reviewed. Two records were incomplete and excluded from analysis. The demographics, application of surveillance definitions, management and outcomes are summarized in Table I.
A significant difference in viral aetiology was observed between adult and paediatric patients (p<0.001). The number of patients with more than one virus detected or mixed viral/pathogenic respiratory bacteria detection were similar (adult [17, 33.3%] vs. paediatric, [23, 39%], p=0.57). Only four adults (7.8%) and three (5.1%) paediatric viral infections were hospital-acquired (respiratory symptoms ≥72 hours after admission), none were identified as influenza A or B. The majority of patients admitted to ICUs met the clinical definition of acute respiratory illness (ARI), however, less than one-third met either the WHO or CDC influenza-like illness (ILI) definitions (Table. 1) [5]. No difference was noted between adult and paediatric patients. A total of 23 (39%) paediatric patients had an infectious disease consult compared to only 12% of adult patients (n=6) with positive respiratory specimens (p=0.002).
Influenza vaccination history in the current season was documented in 3.9% (n=2) of adults and none of the paediatric vaccine-eligible group (p=0.001). Empiric antiviral agents were used 23 (45.1%) and nine (15.3%) of adults and paediatric patients respectively. Most antivirals were started within 72 hours of symptom onset.
Finally, only 42.3% of patients had documented infection control (IC) orders. There was no significant difference between the number of orders placed in community facilities vs. tertiary facilities (30.8% vs. 45.9%, p=0.17), however, there was a difference between the number of orders meeting guidelines between the types of facilities, i.e., the appropriate precautions were applied to the syndrome or pathogen being queried during admission (e.g., contact and droplet precautions for the investigation of viral respiratory tract infections). The community hospital orders met guidelines in 12.5% of orders compared to 92.3% of orders in teaching hospitals (p=0.001). Among children, 34 (58%) had IC orders, 31 (92%) met infection control guidelines. Among adults, 11 (22%) had orders, and four (36%) met guidelines.
DISCUSSION AND CONCLUSION
Viral respiratory infections are a common cause of death in North America. It is estimated that influenza alone is responsible for approximately 12,200 hospitalizations and 3,500 deaths in Canada annually [6]. Our multi-centre study shows significant differences in epidemiological, microbiological and clinical management of adults and pediatric patients in whom a diagnosis of vRTI is queried.
Respiratory viruses elicit similar symptom profiles from the individuals. To date, there have been several prospective cohort studies to determine the performance of case definitions in the hospital setting [5]. However, these surveillance definitions are not intended to be applied for clinical case management. Our study shows that ARI may be a useful definition when considering a respiratory viral infection in the intensive care unit, and maybe a useful tool for improved case finding. This study also showed that the majority of care providers did not document evidence of vaccination in patients with detectable vRTIs and only provided antivirals in a limited number of cases; a finding that has been corroborated by other studies [7,8]. Finally, there was limited documentation of infection control protocols on ICUs. Just over half of paediatric charts had documented evidence compared to less than one-quarter of adults and fewer than 36% of adults had orders matching regional guidelines.
This study is limited by its retrospective nature and small sample size. Viral testing was not systematic and may have varied by site and between children and adults. In addition, we were unable to determine what level of preventative measures were performed retrospectively if undocumented. Vaccination and infection control protocols are implemented at the provincial and institutional level and variations may occur. The strength of our study is that the infection prevention and control policy is standardized across our health region. However, vaccination documentation and documentation of infection control protocols could be included as part of ICU checklists across all health regions and individual hospitals, which would lead to improved case management and harmonization IPAC control measures.
vRTIs are common with detection increasing within the ICU setting. Our study showed differences in vRTIs between adult and paediatric patients and detected hospital-acquired transmission was noted to be uncommon. Infection control orders were sub-optimally documented in ICUs. Healthcare providers are the link to promoting vaccine uptake, appropriate prescribing of antimicrobials and mitigation of viral propagation. This study highlights the need for further research into respiratory viral illness in ICUs, and barriers with regard to the implementation of infection control measures especially in light of rapidly changing viral burdens. Moreover, the present observations could be compared with other Canadian or other worldwide hospitals.
REFERENCES
1. Costello M, Sabatini LM, Yungbluth M. Chapter 55: Viral Infections. 22 ed: Elsevier Canada; 2011.
2. Kelesidis T, Mastoris I, Metsini A, Tsiodras S. How to approach and treat viral infections in ICU patients. BMC Infect Dis. 2014;14:321.
3. Stiver HG, Evans GA, Aoki FY, Allen UD, Laverdière M. Guidance on the use of antiviral drugs for influenza in acute care facilities in Canada, 2014-2015. Can J Infect Dis Med Microbiol. 2015;26(1):e5-8.
4. Health Canada LCfDC, D.vision of Nosocomial and Occupational Infections. Routine practices and additional precautions for preventing the transmission of infection in health care. Can Commun Dis Rep. 1999;25 Suppl 4:1-142.
5. Jiang L, Lee VJ, Lim WY, et al. Performance of case definitions for influenza surveillance. Euro Surveill. 2015;20(22):21145.
6. Flu (influenza): For health professionals. Government of Canada. https:// www.canada.ca/en/public-health/services/diseases/flu-influenza/health-professionals.html. Published 2018. Accessed 2019-03-26, 2019.
7. Petek D, Kamnik-Jug K. Motivators and barriers to vaccination of health professionals against seasonal influenza in primary healthcare. BMC Health Serv Res. 2018;18(1):853.
8. Boey L, Bral C, Roelants M, et al. Attitudes, believes, determinants and organisational barriers behind the low seasonal influenza vaccination uptake in healthcare workers - A cross-sectional survey. Vaccine. 2018;36(23):3351-3358.


