Filip Pajtondziev, MScHQ, CPHI(C), CIC1,2*, Katherine Paphitis, PhD, MSc, CPHI(C), CIC3,4, Jennifer Happe, MSc4,5, and
Rylan Egan, PhD, MEd1
1 Queen’s University, Kingston, Ontario, Canada
2 Brant County Health Unit, Brantford, Ontario, Canada
3 Public Health Ontario, Toronto, Ontario, Canada
4 IPAC Canada Surveillance and Applied Epidemiology Interest Group, Winnipeg, Manitoba, Canada
5 Alberta Health Services, Calgary, Alberta, Canada
*Corresponding author
Filip Pajtondziev
Brant County Health Unit
194 Terrace Hill Street
Brantford, ON, N3R 1G7
Tel: 519-753-4937, ext. 251 | email: This email address is being protected from spambots. You need JavaScript enabled to view it.
ABSTRACT
Background: Carbapenemase-producing Enterobacteriaceae (CPE) infections are difficult to treat and are associated with high mortality. This study investigated admission screening practices for CPE risk factors within Canadian acute care inpatient hospitals (herein referred to as hospitals) and long-term care facilities (LTCF), and identified perceived barriers to screening, as reported by respondents working in these settings. Awareness of perceived barriers can inform improvements to current screening practices.
Methods: An electronic, cross-sectional survey was distributed to a convenience sample consisting of members of the IPAC Canada surveillance and LTCF interest groups, and to the Canadian Nosocomial Infection Surveillance Program’s Carbapenemase-Producing Organisms Workgroup. Recipients with a role in infection prevention and control in a Canadian hospital or LTCF were asked to respond. Survey data were collected from September 7 to December 11, 2020. Descriptive analyses were used to compare the proportion of LTCF and hospital-based respondents who reported that their facility conducted admission screening for CPE risk factors, and to describe perceived barriers to screening.
Results: There was a significant difference between respondents from LTCFs and hospitals as to whether screening was performed for CPE (p<0.001), with hospital-based respondents being more likely to report admission screening. Similarly, there was a statistically detectable difference between respondent facility size (based on number of beds) and whether screening was performed (p=0.039), with admission screening reported more frequently by respondents working in facilities with 250-499 beds. Similar barriers to admission screening were identified by LTCF and hospital-based respondents, with both reporting a lack of resources, staffing, and cost as perceived barriers in their facility. Additionally, LTCF-based respondents reported a lack of policies or processes to guide screening.
Conclusions: Awareness of specific barriers to admission screening for CPE may help hospitals and LTCFs to improve surveillance practices for CPE colonization and infection to inform prompt implementation of IPAC measures that may limit transmission within healthcare settings.
KEYWORDS
Drug resistance, microbial, Enterobacteriaceae, infection control, long-term care, public health surveillance
INTRODUCTION
Carbapenemase-producing Enterobacteriaceae (CPE) are gram-negative bacteria typically found in the gastrointestinal tract. CPE treatment options are limited because CPE bacteria produce the enzyme carbapenemase, which can hydrolyze penicillin, cephalosporin, and carbapenem drugs, and is commonly found on plasmids containing multiple determinants of resistance to other classes of antimicrobials (Falagas et al., 2011). CPE infections are associated with high mortality due to limited treatment options (Iovleva & Doi, 2017). Increasing global
incidence of CPE prompted the World Health Organization to name CPE as a priority antimicrobial resistant pathogen in 2017 (Asokan et al., 2019). CPE was first reported in Canada in 2008, and the Canadian Nosocomial Infection Surveillance Program (CNISP) has been conducting surveillance on CPE in inpatient and outpatient acute care settings since 2010
Mitchell et al., 2020). While initially reported cases were linked to exposure via travel or receipt of healthcare abroad, data from a population-based survey (2007-2015) found that the incidence of CPE infections in south-central Ontario increased over the study period and that many cases likely acquired infection domestically (Kohler et al., 2018).
CPE can be transmitted from person-to-person directly or indirectly, including via poor hand hygiene by healthcare workers, or through sharing of contaminated medical equipment between patients without proper cleaning and disinfection (Ontario Agency for Health Protection and Promotion, 2019). Exposure to CPE within healthcare facilities has been identified as a primary risk factor for infection, with increased length of stay and invasive procedures each increasing the risk of exposure (Epstein et al., 2014; Ontario Agency for Health Protection and Promotion, 2019). If introduced into the environment, including on surfaces or in sink or shower drains, CPE can persist and contribute to indirect transmission of infection (De Geyter et al., 2017; Ontario Agency for Health Protection and Promotion, 2019; Park et al., 2020). Once colonized or infected with CPE, a person can remain colonized and potentially infectious to others for several months to over a year (Ontario Agency for Health Protection and Promotion, 2019).
Active screening to identify patients or residents who are colonized with CPE is key to preventing transmission within healthcare settings. The incidence of healthcare associated CPE infections remains low in Canadian acute care hospitals; however, the limited treatment options and high mortality make it an important pathogen to monitor (Government of Canada, 2017). Best practices suggest that healthcare facilities have a screening program for antibiotic resistant organisms that involves active screening for all admitted patients, including a screening questionnaire for risk factors, followed by culture if risk factors are present (Ho et al., 2012; Ontario Agency for Health Protection and Promotion, Provincial Infectious Diseases Advisory Committee (PIDAC), 2013; Provincial Infection Control Network of British Columbia, 2013). Screening programs allow for early identification of antimicrobial resistant organisms and prompt implementation of infection prevention and control (IPAC) measures to curb transmission.
The primary objectives of this study were to (1) assess whether Canadian long-term care facilities (LTCF) and acute care hospitals (hospitals) conduct CPE admission screening, and (2) to identify barriers to screening for CPE, by surveying individuals with IPAC responsibilities in Canadian hospitals and LTCF.
METHODS
An electronic cross-sectional survey was created using Survey Monkey and included questions on whether CPE admission screening was conducted by the respondent’s employing hospital or LTCF, what risk factors were screened for, and what perceived barriers existed to conducting screening. Survey questions were adapted from a similar survey by Martischang et al. (2019), with additional questions to explore perceived barriers to admission screening for CPE. A copy of the survey is available from the authors upon request. The survey was distributed to the IPAC Canada Surveillance and Applied Epidemiology (SAEIG) and Long-Term Care Interest Groups, and to the CNISP Carbapenemase-Producing Organisms Workgroup.
A convenience sample of respondents working in a Canadian hospital or LTCF with a role in IPAC was asked to complete the survey or to share the survey with the appropriate person(s) within their organization. Survey data were collected from September 7 to December 11, 2020. To maintain anonymity, respondents were not asked to identify their employing facility or to report their specific role, beyond providing information on setting type (hospital vs. LTCF), province, and bed size, so it was possible for multiple responses to be received from more than one respondent in the same facility. There were 66 responses to the survey. For all analyses, hospital or LTCF-based respondents and not their employing facility were the unit of analysis. Statistical significance was calculated between categorical variables using Pearson’s Chi-square tests. For all analyses, the two-tailed statistical significance level was set at 5% (p=0.05).
Ethics approval was received by Queen’s University (TRAQ: 6030268). Descriptive analyses were performed in Microsoft Excel and Stata v12.0 (STATACORP) to assess the proportion of hospitals and LTCF that reported performing screening for CPE, and to compare identified barriers to screening reported by each facility type.
RESULTS
Respondent demographics
There were 66 survey responses from respondents working in Canadian hospitals (63.6%, n=42) and LTCFs (36.4%, n=24), with 65 having sufficient data for analysis. Using cross-tabulation with institutional characteristics we confirmed that our sample represented at least 27 unique hospital settings and 16 LTCF. All hospital-based respondents were from inpatient hospital settings. Responses were most commonly received from Ontario (N=42, 66%), the Prairie provinces (10, 15%), the Atlantic provinces (6, 9%), British Columbia (4, 6%), and Quebec (1, 2%). Respondent facilities ranged in size from <100 to ≥500 beds with most hospital-based respondents from facilities with 250-499 beds (n=17, 40.5%) and most LTCF-based respondents from facilities with 100-249 beds (n=10, 41.7%). One respondent did not provide any information beyond facility type, bed size and geographic location and was dropped from subsequent analyses.
Facility screening practices
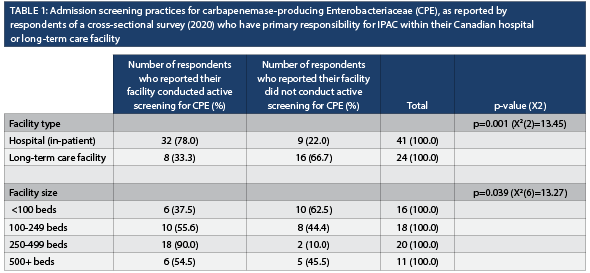
Overall, 61.5% (n=40) of survey respondents reported that their facility conducted admission screening for CPE; significantly more hospital-based respondents reported conducting screening (78.0%, n=32) compared to LTCF-based respondents (33.3%, n=8) (p=0.001) (Table 1). There was a significant difference between facility size and whether or not the facility conducted screening for CPE (p=0.039, Table 1). Of those who conducted screening, 92.1% (n=35 of 38 respondents) reported placing someone with an identified risk factor on contact precautions and 96.8% (n=30 of 31 respondents) reported collecting a rectal specimen for culture to test for the presence of CPE.
Risk factors for which facilities conduct screening
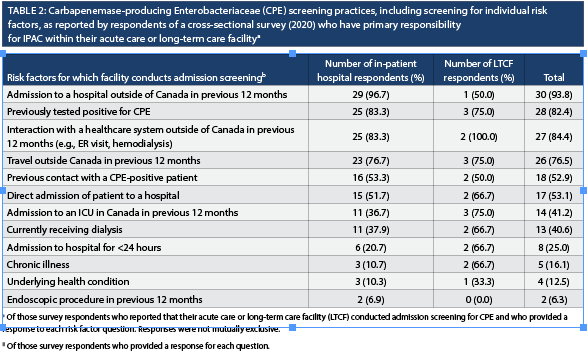
Of those hospital-based respondents who reported that their facility conducted admission screening for CPE, 88.2% (n=30) reported screening for one or more specific risk factors. Risk factor screening variables reported by most hospital-based respondents included international travel, admission to or interaction with a healthcare facility (e.g., hemodialysis or an emergency room visit) outside of Canada in the 12 months prior to admission. (Table 2). Only 4 LTCF-based respondents (50% of LTCF-based respondents who reported that their facility conducted screening) provided information on specific risk factors included in routine screening (Table 2).
Reported barriers to screening
Among the 25 respondents who reported their facility did not conduct active screening for CPE (n=16 LTCF, n=9 hospitals), 22 (n=14 LTCF, n=8 hospitals) identified one or more specific barriers to screening, including a lack of resources (n=11), costs (n=8), and laboratory accessibility (n=5). Although not specifically asked about current processes/policies, nine individuals responding on behalf of a LTCF, voluntarily reported a lack of current policies or procedures for CPE screening as a specific barrier.
Similarly, of those 40 respondents who reported that their facility conducted active screening for CPE, 28 (70%; n=23 hospitals, n=5 LTCF) identified one or more barriers to screening, including a lack of resources (n=12), costs (n=12), and staffing (n=12). Several hospital-based respondents (n=5) voluntarily reported as an additional barrier that there was a lack of awareness at their facility regarding who should be screened for CPE and who had responsibility to conduct screening.
DISCUSSION
Early identification of CPE is important to control the spread of these difficult to treat organisms in healthcare settings. Our survey found that while over half of respondents from Canadian healthcare settings reported that their facility conducted screening for CPE, respondents based in hospital settings were more likely to report that their facility conducted screening than those based in LTCF. Respondents from LTCF reported a lack of policies or processes on screening as a primary barrier to admission screening for CPE, potentially indicating that LTCF may benefit from setting-specific guidance for CPE screening. The finding that respondents from smaller facilities were less likely to report screening for CPE than their larger counterparts may have been related to reported constraints such as a lack of resources, or costs of screening.
Recent studies suggest that CPE acquisition in Canada is increasingly occurring through local nosocomial transmission, although travel and healthcare outside of Canada remain important risk factors (Kohler et al., 2018; Mitchell et al., 2020). Previously testing positive for CPE, international travel and receiving healthcare outside of Canada, including hospitalization, an Emergency Room visit, and hemodialysis, were the most commonly reported risk factors respondents screened for, while few reported screening for hospitalization or short-term admission (<24 hours) in Canada as risk factors. Screening for nosocomial exposure within Canada is important to identify locally acquired cases of CPE and to ensure IPAC measures are promptly implemented.
Among respondents whose facilities screened for CPE, most acted on the information gathered during the risk assessment by implementing additional precautions, and collecting a specimen for culture if one or more risk factors were identified through admission screening. A previous study on the universal screening for CPE by Lapointe-Shaw et al. (2017) found that the patient screening process prevented the transmission of six CPE colonizations per 1,000 patients, three CPE infections per 10,000 patients and seven deaths per 100,000 patients. Overall, the study concluded that universal screening was cost effective and even cost saving, contingent on the number of CPE colonized patients identified on admission (Lapointe-Shaw et al., 2017). A recent Swiss study identified that a lack of national standards for screening, and not having a clear understanding of relevant risk factors for infection were specific barriers to admission screening for multi-drug resistant organisms in Swiss healthcare facilities, including CPE (Martischang et al., 2019).
A major limitation of this study was that all analyses were respondent-based and not institution-based. As participants were not asked to identify their particular facility, it is plausible that more than one survey response was received for a particular hospital or LTCF. This may have contributed to overrepresentation of some facilities within the data, which was not controlled for in our analyses. This may have artificially inflated the proportion of a particular facility type that reported screening. Similarly, the importance of individual barriers may have been overestimated in either or both settings. As the bulk of responses were received from individuals working in Ontario healthcare settings, this may limit the external generalizability of our findings to healthcare settings in other provinces, and particularly where provincial, territorial or local guidance for CPE surveillance exists (if applicable). Another limitation included the survey representing a small proportion of facilities in Canada.
Common barriers to conducting CPE screening reported by respondents working in Canadian healthcare settings included a lack of resources, staff, cost, and a protocol to guide the process and establish accountability. Strategies to address these barriers are important to support consistent screening across Canada as a means of CPE prevention and control. Consideration of risk factors that may increase the risk of local exposure to CPE can support prompt detection of CPE colonization and infection, further supporting the effectiveness of admission screening as a means of limiting nosocomial transmission.
REFERENCES
Asokan, G., Ramadhan, T., Ahmed, E., Sanad, H. (2019).
WHO Global Priority Pathogens List: A bibliometric analysis of Medline-PubMed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Medical Journal, 34(3), 18 4193. https://doi.org/10.5001/omj.2019.37.
De Geyter, D., Blommaert, L., Verbraeken, N., Sevenois, M., Huyghens, L., Martini, H., et al. (2017). The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrobial Resistance & Infection Control, 6(1), 24. https://doi.org/10.1186/s13756-017-0182-3.
Epstein, L., Hunter, J. C., Arwady, M. A., Tsai, V., Stein, L., Gribogiannis, M., et al. (2014). New Delhi metallo-β-lactamase–producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. Journal of the American Medical Association, 312(14), 1447. https://doi.org/10.1001/jama.2014.12720.
Falagas, M. E., Karageorgopoulos, D. E., & Nordmann, P. (2011). Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiology, 6(6), 653–666. https://doi.org/10.2217/fmb.11.49.
Government of Canada. (2017). Routine practices and additional precautions for preventing the transmission of infection in healthcare settings [Internet]. Ottawa, ON: Government of Canada. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/routine-practices-precautions-healthcare-associated-infections/part-a.html#A.III.B.
Ho, C., Lau, A., Cimon, K., Farrah, K., & Gardam, M. (2012). Screening, isolation, and decolonization strategies for Vancomycin-Resistant Enterococci or Extended Spectrum Beta-Lactamase Producing Organisms: a systematic review of the clinical evidence and health services impact. Canadian Agency for Drugs and Technologies in Health. http://www.ncbi.nlm.nih.gov/books/NBK174613/.
Iovleva, A., & Doi, Y. (2017). Carbapenem-Resistant Enterobacteriaceae. Clinics in Laboratory Medicine, 37(2), 303–315. https://doi.org/10.1016/j.cll.2017.01.005.
Kohler, P. P., Melano, R. G., Patel, S. N., Shafinaz, S., Faheem, A., Coleman, B. L., et al. (2018). Emergence of carbapenemase-producing Enterobacteriaceae, South-Central Ontario, Canada. Emerging Infectious Diseases, 24(9), 1674–1682. https://doi.org/10.3201/eid2409.180164.
Lapointe-Shaw, L., Voruganti, T., Kohler, P., Thein, H.-H., Sander, B., & McGeer, A. (2017). Cost-effectiveness analysis of universal screening for carbapenemase-producing Enterobacteriaceae in hospital inpatients. European Journal of Clinical Microbiology & Infectious Diseases, 36(6), 1047–1055. https://doi.org/10.1007/s10096-016-2890-7.
Martischang, R., Buetti, N., Balmelli, C., Saam, M., Widmer, A., & Harbarth, S. (2019). Nation-wide survey of screening practices to detect carriers of multi-drug resistant organisms upon admission to Swiss healthcare institutions. Antimicrobial Resistance & Infection Control, 8(1), 37.
https://doi.org/10.1186/s13756-019-0479-5.
Mitchell, R., Mataseje, L., Boyd, D., Al-Rawahi, G., Davis, I., Ellis, C., et al. (2020). A growing concern: the emergence and dissemination of carbapenemase-producing Enterobacterales (CPE) in Canada. Infection Control & Hospital Epidemiology, 41(S1), s454–s454. https://doi.org/10.1017/ice.2020.1126.
Ontario Agency for Health Protection and Promotion, Provincial Infectious Diseases Advisory Committee (PIDAC). (2013). Annex A: screening, testing and surveillance for antibiotic-resistant organisms (AROs) Annexed to: Routine practices and additional precautions in all health care settings [Internet]. Toronto, ON: Queen’s Printer for Ontario.
https://www.publichealthontario.ca/-/media/Documents/A/2013/aros-screening-testing-surveillance.pdf?rev=5b027cecb0034a5e9c3b57635de1dc23&sc_lang=en.
Ontario Agency for Health Protection and Promotion (Public Health Ontario). (2019). Carbapenemase-producing Enterobacteriaceae: Frequently asked questions [Internet]. Toronto, ON: Queen’s Printer for Ontario.
https://www.publichealthontario.ca/-/media/documents/F/2019/faq-cpe.pdf?la=en.
Park, S. C., Parikh, H., Vegesana, K., Stoesser, N., Barry, K. E., Kotay, S. M., et al. (2020). Risk factors associated with carbapenemase-producing Enterobacterales (CPE) positivity in the hospital wastewater environment. Applied and Environmental Microbiology, 86(24), e01715-20. https://doi.org/10.1128/AEM.01715-20.
Provincial Infection Control Network of British Columbia. (2013). Antibiotic resistant organisms prevention and control guidelines for healthcare facilities [Internet]. Vancouver, BC: The Provincial Infection Control Network of BC (PICNet). https://www.picnet.ca/wp-content/uploads/PICNet_ARO_Guidelines_March2013.pdf.


