Marthe Charles, MD, MSc1*, Teresa Zurberg, RCT2, Tracey Woznow, BSc, BEd2, Eric Eckbo, MD1, Lale Asku, BA2,
Roy Pescador, BSc2, Leonardo Gomez Navas, BA2, Robert O’Neill, BA3, Esther Thompson, BSc, RPN2, and Elizabeth Bryce, MD1
1 Division of Medical Microbiology and Infection Control, Vancouver Coastal Health, Vancouver, Canada
2 Quality, Patient and Safety, and Infection Prevention and Control, Vancouver Coastal Health, Vancouver, Canada
3 Resident Services, Long-term care, Vancouver Coastal Health, Vancouver, Canada
*Corresponding author:
Dr. Marthe Charles
Division Head
Medical Microbiology and Infection Prevention
Vancouver General Hospital
JPN 1110 - 899 West 12th Avenue
Vancouver, British Columbia V5Z 1M9
Tel: 604-875-4547 | Fax: 604-875-4359
ABSTRACT
Background: Long-term care (LTC) residents have been disproportionately affected by COVID-19 with higher morbidity and mortality. The use of canines for the detection of COVID-19 has been successful in controlled laboratory settings. They have the potential to provide rapid, non-invasive screening in congregate living settings. The ability to assess whether laboratory-trained canines can transfer their scent detection skills to the clinical settings has had limited evaluation.
Methods: At Vancouver Coastal Health, two canines were previously trained and validated to differentiate COVID-19 positive and negative PCR samples from breath, sweat and gargle clinical samples from scent stands. Subsequently, they were taught to alert from pillowcases. Following successful validation and in collaboration with a local LTC, the canines performed weekly blind screening of residents’ pillowcases during the Omicron wave.
Results: A third-party, double-blind validation on pillowcases demonstrated an overall sensitivity of 100%. The specificity was 100% for the first canine and 82.6% for the second canine. Although no clinical cases occurred during the six-week pilot project, the agreement between the two canine teams was 98.4% on room alerts. In addition, the two teams were able to seamlessly transfer their laboratory skill sets to the LTC setting.
Conclusion: Third-party evaluation determined that canines could successfully be trained to detect COVID-19 from pillowcases. The canine teams were then able to efficiently shift their skill set from the laboratory into an operational setting. This pilot project supports that the deployment of canine detection in a clinical setting is possible and could be considered in congregate living settings.
KEYWORDS
Canine bio-detection, COVID-19, field detection
INTRODUCTION
Globally, countries are now stepping down COVID-19 precautions, however, screening and diagnostic protocols for identification and management (including early treatment) in healthcare settings are still required. The latter includes vulnerable populations in congregate settings such as long-term care, assisted living and group homes where transmission is rapid and morbidity significant.
Long-term care (LTC) residents have been disproportionately affected by COVID-19 with higher morbidity and mortality (Cronin & Evans, 2022). Rapid antigen testing for viral detection have been useful but are invasive and lack sensitivity particularly during the asymptomatic phase (Indelicato, Mohamed, Dewan, & Morley, 2022). Currently, early identification of local LTC outbreaks employs molecular testing and screening of symptomatic individuals using nasopharyngeal or gargle samples. This requires invasive sample collection that can be labour intensive in congregate settings and secondary independent laboratory confirmation is still required. (Yen, Schwartz, & Hsueh, 2022).
Canines have shown remarkable promise in identifying COVID-19 from clinical samples with high sensitivity and specificity and the authors – in collaboration with Health Canada successfully trained canines to accurately detect positive COVID‑19 patients with an overall sensitivity and specificity of 100% and 87.5% respectively (Charles et al., 2022). Numerous groups around the world have demonstrated that canines can efficiently be trained for COVID-19 detection (Dickey & Junqueira, 2023). Some researchers have demonstrated that trained canines have the capability to detect pre-symptomatic and asymptomatic infections and even differentiate between COVID-19 and other circulating respiratory viruses (i.e., Influenza, Parainfluenza, RSV or Adenovirus) (Grandjean et al., 2020; N. A. Ten Hagen et al., 2021; Nele Alexandra Ten Hagen et al., 2022). However, concerns were raised regarding the feasibility and timing of the deployment of COVID-19 bio-detection canine screening teams specifically with regard to logistical and operational considerations, human resource requirements, biohazard risk and overall practicality (Otto et al., 2023).
To date, no other group has described the use of environmental screening in congregate settings and the steps necessary for successful transition from laboratory to clinical settings has not been well studied (D’Aniello et al., 2021; Vesga et al., 2021). Previous experience in hospital environmental screening for the detection of Clostridioides difficile (Charles et al., 2019) led to the decision to train canines to detect COVID-19 from pillowcases. COVID-19 environmental screening by populationnon-invasive screening in congregate settings such as long-term care where the risk of transmission and serious outcomes is significantly higher than in the general population (Cronin & Evans, 2022).
This article describes the methodology and validation results of two canines trained to detect COVID-19 from a unique source – pillowcases, and details the successful transfer of their laboratory skills to the LTC setting.
METHODS
Training samples
Canines (two Labradors (a male and a female), both four years old) were previously trained to detect only COVID‑19 infections from gargle, sweat and surgical masks, and were not used for any other scent detection (Charles et al., 2022). Care was taken to include samples from patients found in acute and long-term care of various ages and sexes and infected with B.1.1.7 (alpha) variants and strains with P.1 (gamma) or B1.617.2 (delta) mutations. During the five-month period of sample collection, epidemiology indicated that alpha and gamma variants comprised between 40 to 60% of circulating strains while the delta variant accounted for 15%. COVID-19 status was determined by nucleic acid amplification [Polymerase Chain Reaction (PCR)] and positive participants were deemed eligible if their first positive COVID‑19 test occurred within a 10-day period from symptom onset. Negative patient samples were used as training control materials. Patients were required to have tested negative for Influenza A/B, RSV and COVID-19 within 48 hours of sample collection. Routine COVID-19 testing targeted the envelope (E) gene and the RdRP gene of SARS-CoV-2.(Corman et al., 2020).
Odour preparation for pillowcases
Pillowcases were chosen due to their ability to harbour all the components (breath, saliva, sweat) that the canines were previously trained and validated on. Selected patients had their pillowcases collected within 48 hours of their positive PCR test. The pillowcase had to be in use for at least 24 hours to be suitable for screening. When more than one pillowcase was available, the pillowcase found under the head of the patient was preferred. All pillowcases were stored in sealed plastic bags and kept at 4°C to preserve the scent profile.
Canine training & evaluation
Prior to this pilot study, the canines were previously trained and validated to detect COVID-19 from masks, gargles and nasopharyngeal samples over a 19-week period by one trainer (Charles et al., 2022). As described above, pillowcases were selected for environmental screening over a two-week period. Canines were successfully trained to alert on pillowcases from COVID‑19 positive patients’ items. The canines were conditioned to alert to a positive odour with a freeze and stare response until acknowledgement by the handler. All the training and evaluation sessions of the canines took place in a controlled setting at the canine training center and were video recorded.
Pilot project
Phase I validation of the canine scent detection team on pillowcases. A double-blind, randomized formal evaluation by a third-party professional canine scent detection handler was performed over one day using a new set of 12 pillowcase odours (seven positive, five negatives), on which the canines were not previously trained. Each run consisted of 10 odour stands (Figure 1). The first stand in each line-up did not contain an odour. Each run contained two to three COVID-19 positive odours, a maximum of three COVID-19 negative odours, and a variety of associated odours representing potentially distracting scents that the canines might encounter during a training session (clean unused pillowcases, unused latex gloves, and accelerated hydrogen peroxide cleaning wipes). Acceptability criteria for a successful validation of a screening method was set at a positive percentage agreement (PPA) and negative percentage agreement (NPA) above 80%.
Pilot project – Phase II deployment in LTC
The third floor of a local 156-bed LTC, affiliated with Vancouver Coastal Health, was selected for this phase. The facility had a majority of shared rooms. Prior to the start of the pilot, members of the canine detection program met with the third-floor residents’ council, described to them the aims of the pilot, what was involved, answered any questions and asked for their participation in this quality improvement project. In addition, the Health Career Access Program (HCAP) that provides a path for applicants with no health care experience to be hired and receive paid employer sponsored healthcare assistant training as part of their employment (Vancouver, 2021) agreed to have students partipate in this pilot project. The terms of the study were reviewed and approved by local public health authorities and was subject to current public health COVID-19 local guidelines.

Screening methodology
The screening team was composed of the canines and canine detection specialist (CDS), an Infection preventionist, a research assistant and a nurse manager. Two canines (Labradors) and two CDS were deployed for each of the screenings. Screening was conducted weekly using one disposable non-woven laundry bag for pillowcases per room per week. Investigators and HCAP students went from room to room using routine infection precautions to change the pillowcases of each resident. The used pillowcases from residents in the same room were then batched and placed into a disposable laundry bag that was hung outside that specific residents’ room on the handrail (Figure 2). Residents sharing a room, as per local infection prevention and control practices would all be considered as exposed in the event of a positive case; and batching allowed for faster screening. Hand hygiene and donning of new gloves was done between handling laundry bags to avoid odour cross contamination. At the end of the screening, the assigned unit staff disposed of the dirty pillowcases in the laundry as per their local process and the used disposable laundry bags were discarded in a regular garbage bin.
Prior to each screening, the canine team reviewed the census of known positive cases of COVID-19 in the unit as a cluster event. The floor was divided in two sides. Each CDS and their canine would search one side at a time, any alerts were noted, and then the team proceeded to the other side. The CDS were blinded to the findings of the other canine team. Before the beginning of the screening, the order of the canine screenings was randomized. Each screen was preceded by a quality control run using a PCR-positive COVID-19 training aid.
In the case of positive alerts, (both canines having alerted on the same laundry bag) the infection preventionist assessed the residents in that room for symptoms. In the event that all residents in the room were asymptomatic, a review of symptoms twice daily for five days was initiated. De-identified results of the symptom checks were relayed to the canine detection program to complete the dataset. If a symptomatic resident was identified in the room, PCR testing would follow, and the entire room would be placed on droplet and contact precautions until results would be available. If the PCR results were negative, additional precautions would be discontinued.
Statistical analysis
Both canines in turn, were deployed consecutively for each screening run to enable the collection of interrater reliability data and any incidences of non-concordance between the canines’ alerts. Screening runs were video recorded for quality control purposes and transparency as to how the screening was conducted. Results were recorded and analyzed using Excel 2016 (Microsoft Office) and the categorical agreement, analytic sensitivity, and specificity was calculated based on comparison to the real-time PCR results; calculations were based on a COVID-19 population prevalence of 5% to reflect pandemic prevalence in congregate settings as reported from early estimates from outbreaks in long-term care facilities. Confidence Intervals were calculated using “exact” Clopper-Pearsons CIs. Inter-rater reliability was calculated using GraphPad Prism v9.1.0.
Ethics
The study was reviewed by the University of British Columbia Clinical Research Ethics Office and deemed to fall within the scope of a quality improvement initiative; consequently, only verbal consent was sought to collect disposable surgical masks (breath odours), sweat/body odours and pillowcases. Archived gargle samples already submitted for diagnostic purposes were also used for training purposes.
RESULTS
Pilot project
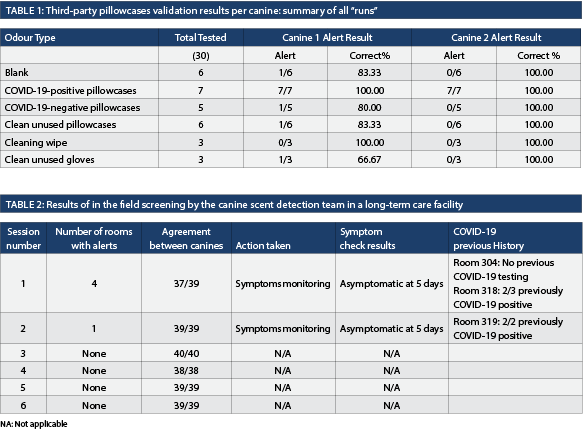
Phase I validation of the canine scent detection team on pillowcases. An initial quality control run of 10 stands was set up, containing only one positive pillowcase and nine blank stands without odours and both canines exhibited 100% sensitivity and specificity. For the official third-party assessment, three runs of 10 metal containers loaded with a combination of positive, negative and associated odours were used (Table 1). The first run included pillowcases from hospitalized patients in an acute care center. The second run included pillowcases from outpatients, and the last run was a mix of both. The first canine showed a positive percent agreement of 100% and a negative percent agreement of 82.61%. The second canine had a 100% positive percent agreement and negative percent agreement. The overall sensitivity and specificity for both canines were 100% and 82.61% respectively. The Kappa agreement was 0.689 (95CI 0.420-0.959) indicating substantial agreement between the canines. Importantly, both canines had no major errors (i.e., missing a positive pillowcase).
Pilot project – Phase II deployment in LTC
From November 10 to December 15, 2022, the two canine teams conducted six screens of the LTC residence on the third floor. The canines had 98.4% agreement on alerts of the rooms screened over the six screening sessions (Table 2). No outbreaks, clusters or new cases occurred during the observation period. Set-up time to reporting decreased from 1.5 hours to 50 minutes for a floor of 22 rooms (41 residents) during the course of the pilot.
DISCUSSION
Screening strategies that assess individuals for evidence of COVID-19 disease provide only a partial picture of epidemiology and are a potentially costly means of identifying cluster events (Crowe et al., 2021). Further, screening in congregate settings such as LTC often consists only of assessing symptomatic individuals even though asymptomatic cases with significant viral shedding have been well documented (Lee et al., 2020).
The deployment of canines in the field can be quite challenging with two principal sampling methods described in the literature: 1) collecting samples to be presented to the canines immediately (sweat, mask, saliva), or 2) direct detection with individuals in a line-up scenario. These methods can be quick but require a large number of staff and/or a dedicated area to setup scent stands. These methods have been assessed and reported from airport (Helsinki), healthcare centers, public transportation systems, concerts and school. (Dickey & Junqueira, 2023).
This pilot study is the first reported use of canine scent detection teams in a LTC facility. Mass screening of wards in LTC with cluster events generally require significant human resources that are often diverted from other necessary services. Canine scent detection teams have been demonstrated to be remarkably successful in rapidly and efficiently identifying COVID-19 from clinical samples. In addition to nasopharyngeal and gargle samples, they have been trained to accurately and quickly screen personal items such as masks, socks and T-shirts (Guest et al., 2022). However, their role in environmental screening has not been explored particularly in congregate live-in situations and has not previously been described as a mean for screening.
In this study, pillowcases were chosen as the environmental item of interest for screening purposes. Pillowcases have prolonged contact with skin, respiratory and oropharyngeal secretions, they can easily be batched or assessed individually and the logistics of conducting a canine search are straightforward. Identification of COVID-19 from a pillowcase could quickly and easily pinpoint not only the geographic area but also the specific exposed patient or resident group. Canine detection for COVID-19 could be easily implemented as a two-stage screening process (e.g., wastewater sampling with canine assessment of pillowcases) and/or a regular screening program (as detailed in our methods section) combined with a diagnostic test (Crowe et al., 2021).
In the laboratory setting, the canines readily adapted to interrogating pillowcase samples exposed to patients with a variety of COVID-19 strains. These encouraging results led to implementation of the clinical phase of this pilot project. Unfortunately, the current shortage of healthcare practitioners constrained the scope of phase two to only screening one LTC floor for six weeks and thus only limited observations were possible. Despite this, the canines demonstrated unequivocally that they could “context shift” their detection skills from the laboratory to a real-life LTC setting. The team was able to develop and implement a deployment plan that could be easily transferable to other congregate settings (e.g., dorms, cruise ships). In addition, the number of resources required, and length of time needed to conduct a screening was refined during the pilot and it would be possible to transfer this concept to other target odours/pathogens in the future.
Both canines investigated all of the laundry bags hung in the hallway during screening. During the pilot project, there were no known cases of COVID-19. The canines alerted to the same rooms (304,318, 319) but unfortunately, further investigation as to what they may have been alerting to was restricted to testing only symptomatic residents. (Public Health had requested that the team conform to the current COVID-19 testing guidelines and not test asymptomatic residents). The authors were, therefore, unable to evaluate whether the alerts were correct or not. Interestingly, both of the canines alerted on the pillowcases of residents who had previously tested positive for COVID-19 weeks before the pilot project. There have been several studies confirming long-term, low-level shedding of the virus and it is possible that the canines’ alerts reflected this phenomenon (Vibholm et al., 2021).
The canines were completely concordant as to all the negative searches and the warm-up quality control runs over the six-week pilot was 100% accurate in the detection of the positive COVID‑19 samples that were hidden. A longer time period and one that permitted COVID-19 testing of all residents would be needed to gain the required amount and quality of data to truly evaluate specificity, sensitivity and inter-rater reliability.
All staff encounters with the CDS teams were positive with healthcare workers exhibiting keen interest in the project and the canines. An infection control practitioner was present during the screenings and fielded questions from environmental services healthcare staff and care aid students about chains of transmission and best practices to keep themselves, residents, and visitors safe. These “in the moment” learning opportunities are valuable and a well-recognized benefit in a healthcare-based screening program (Cheng et al., 2019).
CONCLUSION
A canine scent detection program is valuable when it has the ability to train and test canines on a target pathogen, critically evaluate them, and then shift from the laboratory into a clinical/operational setting. A multidisciplinary group that encompasses expertise from the fields of medical microbiology, environmental infection control, and canine scent detection enables the creation of a team that is credible, reproducible and reliable. The validation process detailed in this article demonstrates the scientific rigour and the acceptable sensitivity and specificity to detect COVID-19. The pilot project further supports that deploying canine detection teams to triage a clinical setting is possible and could, in the future, be used for other congregate living settings and diseases of interest.
REFERENCES
Charles, M., Eckbo, E., Zurberg, T., Woznow, T., Aksu, L., Navas, L. G., Bryce, E. (2022). In search of COVID-19: The ability of biodetection canines to detect COVID-19 odours from clinical samples. Journal of the Association of Medical Microbiology and Infectious Disease Canada, 7(4), 343-349. doi:10.3138/jammi-2022-0017.
Charles, M., Wang, Y., Zurberg, T., Kinna, J., & Bryce, E. (2019). Detecting Clostridioides (Clostridium) difficile using canine teams: What does the nose know? Infection Prevention in Practice, 1(1), 100005.
Cheng, L., Zurberg, T., Kinna, J., Acharya, K., Warren, J., Shajari, S., Bryce, E. (2019). Using scent detection dogs to identify environmental reservoirs of Clostridium difficile: Lessons from the field. Canadian Journal of Infection Control, 34(2), 93-95. Retrieved from https://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=137994639&site=ehost-live.
Corman, V. M., Landt, O., Kaiser, M., Molenkamp, R., Meijer, A., Chu, D. K., Schmidt, M. L. (2020). Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance, 25(3), 2000045.
Cronin, C. J., & Evans, W. N. (2022). Nursing home quality, COVID-19 deaths, and excess mortality. Journal of Health Economics, 82, 102592. doi:10.1016/j.jhealeco.2022.102592.
Crowe, J., Schnaubelt, A. T., Schmidtbonne, S., Angell, K., Bai, J., Eske, T., Broadhurst, M. J. (2021). Assessment of a Program for SARS-CoV-2 Screening and Environmental Monitoring in an Urban Public School District. Journal of the Amercian Medical Association Network Open, 4(9), e2126447. doi:10.1001/jamanetworkopen.2021.26447.
D’Aniello, B., Pinelli, C., Varcamonti, M., Rendine, M., Lombardi, P., & Scandurra, A. (2021). COVID sniffer dogs: technical and ethical concerns. Frontiers in Veterinary Science, 662.
Dickey, T., & Junqueira, H. (2023). COVID-19 scent dog research highlights and synthesis during the pandemic of December 2019-April 2023. Journal of Osteopathic Medicine. doi:10.1515/jom-2023-0104.
Grandjean, D., Sarkis, R., Lecoq-Julien, C., Benard, A., Roger, V., Levesque, E., Desquilbet, L. (2020). Can the detection dog alert on COVID-19 positive persons by sniffing axillary sweat samples? A proof-of-concept study. PLoS One, 15(12), e0243122. doi:10.1371/journal.pone.0243122.
Guest, C., Dewhirst, S. Y., Lindsay, S. W., Allen, D. J., Aziz, S., Baerenbold, O., Davis, J. (2022). Using trained dogs and organic semi-conducting sensors to identify asymptomatic and mild SARS-CoV-2 infections: an observational study. Journal of Travel Medicine, 29(3). doi:10.1093/jtm/taac043.
Indelicato, A. M., Mohamed, Z. H., Dewan, M. J., & Morley, C. P. (2022). Rapid Antigen Test Sensitivity for Asymptomatic COVID-19 Screening. PRiMER, 6. doi:10.22454/primer.2022.276354.
Lee, S., Kim, T., Lee, E., Lee, C., Kim, H., Rhee, H., Kim, T. H. (2020). Clinical Course and Molecular Viral Shedding Among Asymptomatic and Symptomatic Patients With SARS-CoV-2 Infection in a Community Treatment Center in the Republic of Korea. Journal of the Amercian Medical Association Internal Medicine, 180(11), 1447. doi:10.1001/jamainternmed.2020.3862.
Otto, C. M., Sell, T. K., Veenema, T. G., Hosangadi, D., Vahey, R. A., Connell, N. D., & Privor-Dumm, L. (2023). The Promise of Disease Detection Dogs in Pandemic Response: Lessons Learned From COVID-19. Disaster Medicine and Public Health Preparedness, 17(1-6). doi:10.1017/dmp.2021.183.
Ten Hagen, N. A., Twele, F., Meller, S., Jendrny, P., Schulz, C., von Kockritz-Blickwede, M., Volk, H. A. (2021). Discrimination of SARS-CoV-2 Infections From Other Viral Respiratory Infections by Scent Detection Dogs. Frontiers in Medicine (Lausanne), 8(749588). doi:10.3389/fmed.2021.749588.
Ten Hagen, N. A., Twele, F., Meller, S., Wijnen, L., Schulz, C., Schoneberg, C., Volk, H. A. (2022). Canine real-time detection of SARS-CoV-2 infections in the context of a mass screening event. British Medical Journal Global Health, 7(11), e010276. doi:10.1136/bmjgh-2022-010276.
Vancouver, C. H. (2021). Health Career Access Program (HCAP). Retrieved from https://careers.vch.ca/about-vch/education-and-development/hcap/.
Vesga, O., Agudelo, M., Valencia-Jaramillo, A. F., Mira-Montoya, A., Ossa-Ospina, F., Ocampo, E., Aguilar, Y. (2021). Highly sensitive scent-detection of COVID-19 patients in vivo by trained dogs. PLoS One, 16(9), e0257474.
Vibholm, L. K., Nielsen, S. S. F., Pahus, M. H., Frattari, G. S., Olesen, R., Andersen, R., Tolstrup, M. (2021). SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine, 64(103230). doi:10.1016/j.ebiom.2021.103230.
Yen, M.-Y., Schwartz, J., & Hsueh, P.-R. (2022). The implications of the COVID-19 pandemic for long term care facilities. Current Opinion in Infectious Diseases, 35(4), 370-377. doi:10.1097/qco.0000000000000849.


