Dina Badawy, PhD, CIC1*, Samar Tahhan, RN, MSc, CIC1, Gabriel Rebick, MDCM, MPH1,
Clayton Mac Donald, MUDr, FRCP(C), D(ABMM), DTM&H2, Jackie Nugent, RN, BScN, CIC1, Qosay Sarabi, A.B.A, A.H.M, P.H.H3,
Roy D’Oliveira, CEM, M.Eng4, and Lorne Small, BSc, MSc, MD1
1 Department of Infection Prevention and Control, Trillium Health Partners, Mississauga, Ontario, Canada
2 Department of Laboratory Medicine and Genetics, Trillium Health Partners, Mississauga, Ontario, Canada
3 Department of Environmental & Hospitality Services, Trillium Health Partners, Mississauga, Ontario, Canada
4 Department of Maintenance & Engineering Services, Trillium Health Partners, Mississauga, Ontario, Canada
*Corresponding author:
Dina Badawy
Infection Control Practitioner
Trillium Health Partners
Credit Valley Hospital
2200 Eglinton Avenue West,
Mississauga, ON L5M 2N1
Canada
Tel: 1-647-534-1264 | email: This email address is being protected from spambots. You need JavaScript enabled to view it.
ABSTRACT
Background: Between January and September 2022, 10 patients who were admitted to the Intensive Care Unit (ICU) for a period of more than 72 hours were found to have carbapenemase-producing Enterobacteriaceae (CPE) in their screening/clinical specimens. No epidemiological association was found among them, and none had received healthcare outside Canada in the preceding 12 months. The study investigated whether sink drains could be a source of CPE transmission.
Methods: A retrospective study was conducted on all patients admitted to the ICU during the surveillance period who tested positive for CPE, alongside a pilot study involving environmental screening cultures collected from hand hygiene (HH) and washroom sink drains in ICU rooms after the patients had been discharged. Information was gathered on CPE risk factors, such as recent travel history or hospitalization outside of Canada, endoscopic retrograde cholangiopancreatography procedures, or hemodialysis within the last 12 months.
Results: During the surveillance period, 15 non-duplicate CPE isolates were obtained from 14 patients in the ICU. Out of the 14, 10 patients were identified after ICU stays of greater than 72 hours. Klebsiella pneumoniae carbapenemase (KPC) was the most identified carbapenemase, found in five (50%) of isolates, followed by three (30%) New Delhi metallo-β-lactamase, and two (20%) Oxacillinase-48 like carbapenemase. In addition, a total of 33 sink drains underwent testing: 24 were HH sinks, while nine were inpatient washrooms. Among the 24 HH sink drains, nine (37.5%) tested positive for CPE, eight of these sink drains (88.9%) had KPC genes, two (22%) had NDM genes, and one (11%) had VIM genes. Among the HH sink drains contaminated by CPE, three (33.3%) out of these nine shared the same species/gene combination (KPC-Citrobacter freundii) as the CPE-positive patient recently discharged from the room.
Conclusion: These findings suggest a potential link between KPC-producing Citrobacter freundii detected in the HH sink drain and a CPE-positive patient. However, the direction of transmission remains unclear. Further research is necessary to analyze the molecular genotyping and validate any potential relatedness among them, in addition, collaborative efforts from the infection prevention and control, microbiology, environmental, and facilities teams are essential to eliminate CPE from HH sink drains.
KEYWORDS
Carbapenemase-producing Enterobacteriaceae, CPE, KPC, sink drain
INTRODUCTION
The increasing prevalence of carbapenemase-producing Enterobacteriaceae (CPE) is a growing global concern that poses a severe threat to modern medicine. Recent reports from the Centers for Disease Control and Prevention, the World Health Organization, and the Public Health Agency of Canada have classified CPE as one of the most urgent antimicrobial-resistance threats facing humans (CDC, 2019; Garner et al., 2015; WHO, 2017).
Healthcare exposure is the primary source for patient acquisition of CPE, and the bacteria can spread between individuals when workers fail to practice hand hygiene (HH) properly, or when medical equipment is shared without proper cleaning and disinfection (Gupta et al., 2011 & Munoz-Price et al., 2013).
Sinks and shower drains can harbour CPE and have been identified as a source of disease transmission in hospitals. If not properly disinfected, colonized sinks can spread CPE to the next person using the room (Kotsanas et al., 2013 & Leitner et al., 2015). In addition, wastewater drainage systems are prone to biofilm formation and are thought to become seeded when sinks or showers are misused as receptacles for patient waste/wastewater (Parkes & Hota, 2018).
The study’s main objective was to investigate sink drains as a potential reservoir of CPE in the Intensive Care Unit (ICU) to provide insights into ways to prevent the spread of CPE in the ICU setting.
METHODS
Trillium Health Partners (THP) is one of Canada’s largest academically affiliated tertiary care hospitals and consists of highly specialized regional programs. It comprises the Credit Valley Hospital (CVH), the Mississauga Hospital, and the Queensway Health Centre. The study was conducted in the ICU at CVH, which serves a catchment area with a high concentration of new Canadians from the Indian subcontinent. The ICU has 24 private rooms with an attached washroom, admitting medical and surgical patients, and the patient-to-nurse ratio is 1:1. Each ICU room has a HH sink in the patient’s room and a handwashing sink in the patient’s washroom. All HH sinks are compliant with the Canadian Standards Association (CSA).
In the ICU, infection prevention and control measures are in place, including active surveillance for adult patients (>18 years old) who are colonized or infected with CPE. Admission screening swabs are collected from patients with known positive CPE, those who have had contact with a CPE patient, received hemodialysis in the past 12 months, have been admitted to an acute healthcare facility outside Canada in the past 12 months, or have been directly transferred from an acute healthcare facility within Canada with at least a 24-hour admission. Furthermore, ICU healthcare workers are trained on adherence to routine practices, including the use of personal protective equipment, HH, equipment cleaning/disinfection between patients, and contact precautions.
The study was part of a pilot to review all positive CPE results from January to September 2022, using data from the EPIC electronic health records (EHR) system, which was implemented on October 10, 2020, at THP. This system provides seamless access to information through a single point of access across all three THP sites, helping to improve the ability to provide excellence in healthcare.
According to the CPE policy followed at THP, heightened awareness will be declared when the count of nosocomial cases in a particular unit is one less than the threshold required to declare an outbreak. Additionally, point prevalence testing is scheduled to be carried out on days zero, seven and 21 following the declaration date. Throughout the surveillance period, a nine-point prevalence testing was conducted as CPE heightened awareness was declared three times.
Each patient with a positive CPE result was enrolled in the study once, and any subsequent detection of different organisms and/or genes were added to their medical chart. In addition, the study also gathered information on potential risk factors for acquiring CPE, including whether the patient had recently travelled, had been hospitalized, or received medical care outside of Canada within the past year, and whether they had undergone Endoscopic Retrograde Cholangiopancreatography (ERCP) or had received hemodialysis in the last 12 months.
Upon identifying patients who tested positive for CPE during the point prevalence testing, environmental swabs were collected from all 24 HH sink drains and from nine selected washroom sink drains in the patient rooms. The nine washroom sink drains were specifically chosen based on their previous occupancy by CPE-positive patients.
When the results returned negative for these nine washrooms sink drains, no additional testing was conducted on the remaining washroom sinks. In cases where a sink drain tested positive for CPE, multiple swabbing attempts were carried out on the same sink drain following treatment until a negative result was obtained.
In addition, regular huddles, rounds, and meetings were consistently conducted with the ICU staff on infection control practices with a focus on proper disposal practices of patients’ body fluids and medication, and the use of an HH sink for hand washing only.
The onsite microbiology laboratory provides carbapenemase-producing organisms (CPO) screening. Patient and environmental screening are performed using a Starswab II™ charcoal swab (Pretium Canada Packaging ULC, Etobicoke, Canada). Patients are screened by rectal swabs, and for environmental screening, a swab is inserted 5-10 cm into the sink drain pressing against the drain wall. Swabs are inoculated onto Colorex™ mSuperCARBA™ (Micronostyx, Ottawa, Canada) and incubated in the dark, in ambient air at 35°C for 18-24 hours. The growth of suspicious colonies is identified using Bruker MALDI-TOF. Any colonies of Acinetobacter spp., Pseudomonas spp., or Enterobacterales are screened using the NG-test Carba 5 (Hardy Diagnostics, Santa Maria, CA) lateral flow assay. Any colonies positive for the targeted genes, including Klebsiella pneumoniae carbapenemase (KPC), Verona integron-encoded MBL (VIM), New Delhi metallo-β-lactamase (NDM), Oxacillinase-48 like carbapenemase (OXA-48), and Imipenemase (IMP) are reported as positive.
RESULTS
During the surveillance period, 15 non-duplicate CPE isolates were detected from 14 patients. The patients’ age range was 26 to 82 years old with a mean age of 65.7. There were four female and 10 male patients.
Out of the 14 patients who tested positive for CPE, four were in the ICU for less than 72 hours when CPE was detected. Among these four patients, two were already known to have CPE. The third was identified as part of the point prevalence testing conducted at that time. The fourth patient had CPE isolated from a clinical specimen during the assessment. None of these four patients had received hospital care outside of Canada or undergone ERCP. One of these patients had received hemodialysis in the last 12 months, and another patient had recently traveled to India. The latter patient was found to have two CPE isolates harbouring NDM and OXA-48-like genes.
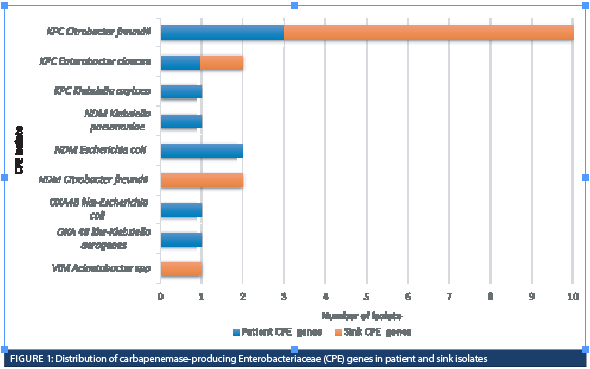
The remaining 10 patients tested positive for CPE after staying in the ICU for more than 72 hours, with a median stay of 28.5 days (ranging from four to 84 days). Among these, three patients were identified through point prevalence testing, four cases resulted from CPE transfer swab upon patient transfer out of the ICU to another unit, and three were isolated from clinical specimens during the disease. Upon their admission to the ICU, seven (70%) tested negative for CPE, while the remaining three patients (30%) did not have their CPE swabs collected. None of these 10 patients had undergone ERCP, or had received healthcare outside Canada in the preceding 12 months. Three out of the 10 patients (30%) had received hemodialysis in the last 12 months, and one had recently travelled to Pakistan.
Among the 10 patient isolates, KPC was the most identified carbapenemase, found in five (50%) of isolates, followed by three (30%) NDM and two (20%) OXA-48-like (Figure 1).
A total of 33 sink drains underwent testing: 24 were HH sinks, while nine were inpatient washrooms. Among the 24 HH sink drains, nine (37.5%) tested positive for CPE (Figure 2). Eight (88.9%) of these sink drains had KPC genes, two (22%) had NDM genes, and 1 (11%) had VIM genes. Out of the nine HH sink drains contaminated by CPE, three (33.3%) shared the same species/gene combination (KPC-Citrobacter freundii) as the CPE-positive patient recently discharged from the room. Within this group of three patients, one had tested negative for CPE upon admission to the ICU room. This patient was undergoing hemodialysis, and the CPE swab was not collected for the other two patients since they didn’t have CPE risk factors.
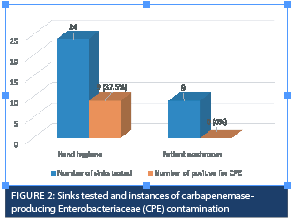
In relation to the remaining five HH sink drains contaminated with the KPC gene, a difference was observed between the gene found in those drains and the gene detected in the CPE-positive patient who had been recently discharged. This difference was noted in three patients. For the other two sink drains, no cases of CPE were identified during the surveillance period, either when patients were present, or after they had been discharged from those two rooms.
We worked closely with the hospital’s environmental department to address the sink drains which were contaminated with CPE. The sinks underwent a total of three treatment rounds using sodium hypochlorite. Initially, 200-300ml of the solution was poured into the drain’s opening and the surrounding area. This step was followed by a 10-minute contact time. Afterward, the sink and drain were rinsed with running water for a minimum of five minutes, ensuring complete removal of any residue. Following a second round of swab collection, certain sink drains were still contaminated with KPC-Citrobacter freundii. We engaged the facilities department to replace P-traps, tailpipes, and drain surface pieces for all contaminated HH sinks. This process took about two weeks to complete. After this remediation, we conducted another round of swab collection to ensure that all sink drains contaminated by CPE were cleared. However, we still found persistent KPC-Citrobacter freundii isolates in two of the sink drains. To deal with this issue, we cleaned the two sink drains with a sporicidal disinfectant following the same methodology of using sodium hypochlorite. After the cleaning process, we took another round of swabs and the results indicated that there was no presence of CPE in the sink drains.
Through our observations during huddles, rounds, and meetings with ICU staff, we became aware of distinct waste disposal practices. These practices involve the disposal of residual fluids from patient treatments – such as saline, enteral feeding bags, ringer lactate, or total parenteral nutrition. If patients undergoing continuous renal replacement therapy experience circuit clotting, staff would empty the remaining fluid from bags containing anticoagulant citrate and dextrose solution into the HH sink. Their rationale is that the fluids are sterile, and the close proximity of the HH sink makes it more convenient for usage compared to the washroom sink located within the patient’s washroom.
DISCUSSION
The study was conducted in the ICU at CVH, which serves a catchment area with a high concentration of new Canadians from the Indian subcontinent. Among hospital-based laboratories, Central West, Toronto Central, Mississauga Halton and Southeast regions had the highest rates of new patients with CPE per 10,000 patients in 2021. Overall rates decreased from 4.2 per 10,000 patients in 2019 to 2.1 per 10,000 patients in 2020 to 2.8 per 10,000 patients in 2021 (Ontario Agency for Health Protection and Promotion & Institute for Quality Management in Healthcare, 2023). Travelling and receiving medical care abroad are significant risk factors for acquiring CPE, and certain countries such as India, Pakistan, and Bangladesh are considered endemic for certain types of CPEs (Ontario Agency for Health Protection and Promotion, 2019). In south-central Ontario, NDM is the most prevalent carbapenemase due to the high volume of travel to and from the Indian subcontinent (Ontario Agency for Health Protection and Promotion, 2019).
Our study shows that among the 10 patient isolates, KPC was the most identified carbapenemase, found in five (50%) of isolates, followed by three (30%) NDM and two (20%) OXA-48-like. Upon review of the CPE isolates from the patients and based on THP policy for CPE , there was no evidence of transmission between patients. Cases were different in CPE genes and were sporadic rather than clustered in adjacent rooms. As a result, the criteria for a confirmed outbreak were not met (Ontario Ministry of Health, 2022).
Several research studies have emphasized the importance of maintaining effective cleaning and disinfection protocols in healthcare settings to prevent the colonization of multidrug-resistant organisms such as CPE in sink drains (De Geyter et al., 2017; Kizny Gordon et al., 2017; Lowe et al., 2013).
A study was conducted at four acute care hospitals in Toronto to investigate the prevalence of CPE inpatient room drains and communal shower drains. The study included the culturing of specimens from 78 patients with CPE, and 356 drains from 178 patient rooms and 17 communal shower rooms. The findings revealed that 14 drains (3.9%) were positive for CPE, and communal shower drains were found to be more contaminated than a sink and bathtub drains. Among the CPE-positive drains, 35.7% had the same species/gene combination as the CPE patient who occupied the room (Jamal et al., 2017).
Another study was conducted in one of the hospitals in Toronto over a period of two years, during which they identified six patients with VIM-producing Enterobacter spp. It was found that all these patients had the same environmental exposure to one of two different rooms in the unit. VIM-producing Enterobacter spp. isolates were discovered from environmental samples taken from the HH sink drains and shower drains of both rooms. The study revealed genetic relatedness among the isolates, and the presence of blaVIM-1 gene was confirmed in all isolates from patients and environmental specimens (Candon et al., 2016).
In our study, we propose a potential link between KPC-producing Citrobacter freundii found in the HH sink drain and a CPE-positive patient. Among the HH sink drains contaminated by CPE, three out of these nine (33.3%) shared the same species/gene combination (KPC-Citrobacter freundii) as the CPE-positive patient recently discharged from the room. Establishing precise clarity regarding the direction of transmission – whether from the patient to the sink or from the sink to the patient – with absolute certainty is a challenging task as none of these three patients occupied a room following a CPE-positive patient, all ICU HH sinks adhere to the CSA standards, ensuring no splashing occurs and, only one out of the three patients tested negative upon admission to the ICU room. Moreover, our findings have unveiled inconsistencies in healthcare workers’ adherence to proper disposal practices. However, a collaborative effort was made between the infection prevention and control team, microbiology team, environmental team, and facilities team to eliminate CPE from the HH sink drains.
Further research is necessary to analyze the molecular genotyping of all CPE-positive isolates and validate any potential relatedness among them. Also, testing the patient’s colonization status with CPE before admission to the ICU would provide valuable insights into potential sources of transmission. It is important to emphasize that this study exclusively focused on the ICU setting and did not encompass other medical or surgical units. Additionally, the study’s restricted sample size and its single-hospital scope may limit the broader applicability of our findings.
In conclusion, due to the increasing prevalence of antimicrobial-resistant organisms, it has become imperative to revise the current CPE policy. This should involve screening all patients upon ICU admission, educating and auditing healthcare workers for compliance with infection control practices, especially focusing on hand hygiene and waste disposal, and establishing a disinfection protocol for hand hygiene sinks in rooms where CPE patients are accommodated.
REFERENCES
Centers for Disease Control and Prevention. (2019). Antibiotic Resistance Threats in the United States. Retrieved from https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
Garner, M.J., Carson, C., Lingohr, E.J., Fazil, A., Edge, V.L., & Waddell, J. (2015). An assessment of antimicrobial resistant disease threats in Canada. PLoS One, 10(4), 1-11. doi:10.1371/journal.pone.0125155
World Health Organization. (2017). Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Retrieved from http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/.
Gupta, N., Limbago, B.M., Patel, J.B., & Kallen, A.J. (2011). Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clinical Infectious Diseases, 53(1), 60-7. doi:10.1093/cid/cir202. PMID: 21653305.
Munoz-Price, L.S., Poirel, L., Bonomo, R.A., Schwaber, M.J., Daikos, G.L., Cormican, M., Cornaglia, G., Garau, J., Gniadkowski, M., Hayden, M.K., Kumarasamy, K., Livermore, D.M., Maya, J.J., Nordmann, P., Patel, J.B., Paterson, D.L., Pitout, J., Villegas, M.V., Wang, H., Woodford, N., & Quinn, J.P. (2013). Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. The Lancet Infectious Diseases, 13(9), 785-796. doi:10.1016/S1473-3099(13)70190-7. PMID: 23969216; PMCID: PMC4673667.
Kotsanas, D., Wijesooriya, W.R., Korman, T.M., Gillespie, E.E., Wright, L., Snook, K., Williams, N., Bell, J.M., Li, H.Y., & Stuart, R.L. (2013). “Down the drain”: carbapenem-resistant bacteria in intensive care unit patients and handwashing sinks. The Medical Journal of Australia, 198(5), 267-269. doi:10.5694/mja12.11757. PMID: 23496403.
Leitner, E., Zarfel, G., Luxner, J., Herzog, K., Pekard-Amenitsch, S., Hoenigl, M., Valentin, T., Feierl, G., Grisold, A.J., Högenauer, C., Sill, H., Krause, R., & Zollner-Schwetz, I. (2015). Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrobial Agents and Chemotherapy, 59(1), 714-716. doi:10.1128/AAC.04306-14. Epub 2014 Oct 27. PMID: 25348541; PMCID: PMC4291428.
Parkes, L.O., & Hota, S.S. (2018). Sink-Related Outbreaks and Mitigation Strategies in Healthcare Facilities. Current Infectious Disease Reports, 20(10). doi:10.1007/s11908-018-0648-3
Ontario Ministry of Health. (2022). Ontario Public Health Standards: Requirements for Programs, Services and Accountability, Infectious Disease Protocol. Appendix 1: Case Definitions and Disease-Specific Information – Disease: Carbapenemase-producing Enterobacteriaceae (CPE) infection or colonization.
Ontario Agency for Health Protection and Promotion (Public Health Ontario); Institute for Quality Management in Healthcare. (2023). Antimicrobial resistance in common hospital pathogens in Ontario: annual laboratory and hospital survey report 2020-21. Toronto, ON: King’s Printer for Ontario.
Ontario Agency for Health Protection and Promotion (Public Health Ontario). (2019). Carbapenemase-producing Enterobacteriaceae: frequently asked questions. Toronto, ON: Queen’s Printer for Ontario.
De Geyter, D., Blommaert, L., Verbraeken, N., et al. (2017). The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrobial Resistance & Infection Control, 6, 24.
https://doi.org/10.1186/s13756-017-0182-3.
Kizny Gordon, A.E., Mathers, A.J., Cheong, E.Y.L., et al. (2017). The Hospital Water Environment as a Reservoir for Carbapenem-Resistant Organisms Causing Hospital-Acquired Infections: A Systematic Review of Literature. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 64(10), 1435-1444. DOI: 10.1093/cid/cix132. PMID: 28200000.
Lowe, C.F., Kus, J.V., Salt, N., Callery, S., Louie, L., Khan, M.A., Vearncombe, M., & Simor, A.E. (2013). Nosocomial transmission of New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae in Toronto, Canada. Infection Control & Hospital Epidemiology, 34(1), 49-55. doi:10.1086/668778. Epub 2012 Nov 20. PMID: 23221192.
Jamal, A., Johnstone, J., Katz, K., McGeer, A., Muller, M., Khan, N., Koren, V., Li, A., Paterson, A., Souto, R., Tang, L., Thomas, C., & Ng, W. (2017). Prevalence of Carbapenemase-Producing Enterobacteriaceae (CPE) in Hospital Drains and Relationship to Patient Isolates in Toronto, Canada. Open Forum Infectious Diseases, 4(suppl_1), S485. https://doi.org/10.1093/ofid/ofx163.1198.
Candon, H., Matukas, L., Patel, S., Melano, R., Tijet, N., Eshaghi, A., McGeer, A., & Johnstone, J. (2016). Transmission of Verona Integron-Encoded Metallo-β-Lactamase-Producing Enterobacteriaceae Over a Two-Year Period Linked to Contaminated Drains. Open Forum Infectious Diseases, 3(suppl_1), 245. https://doi.org/10.1093/ofid/ofw172.112.


